Figure 1.
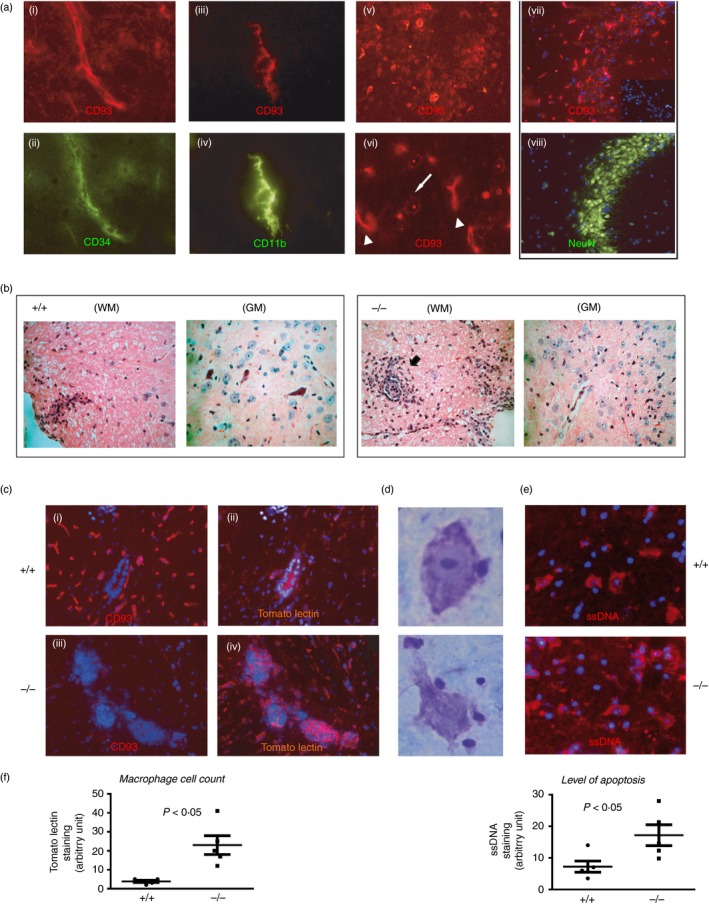
Lack of CD93 confers increased bystander neuronal injury and inflammation in the spinal cord of experimental autoimmune encephalomyelitis (EAE) mice. (a) In a naive C57BL/6 mouse brain, CD93 was expressed on endothelial cells (CD34+), ramified microglia (CD11b+) and neurons (NeuN+ cells). CD93 was also localized in cytoplasmic vesicles in neurons (white arrow in (vi)) compared with a more robust membrane‐restricted CD93 staining in endothelium (arrowhead in (vi)). Representative data obtained from tissue sections from the brains of two animals. (b) Histological haematoxylin analysis of semi‐serial paraffin sections from the spinal cord of representative CD93+/+ and CD93−/− mice after EAE. Cellular infiltrates were prominent in white matter (WM) lesions of CD93+/+ mice whereas these infiltrates where more numerous and localized at the border between the WM and the grey matter (GM) in CD93−/− mice. (c) Immunostaining of EAE (days 15–18) spinal cords. Tomato lectin (TL) identified the infiltrating and reactive macrophages/microglia in CD93+/+ (b) in response to EAE and these cells were more numerous and aggregated at the border between the WM/GM in CD93−/− spinal cords following EAE (d). (d, e) Necrotic‐like Nissl+ neurons (surrounded by many lymphocyte‐like cells) were more numerous in CD93−/− sections and may correspond to cells in late apoptosis as detected by the anti‐ssDNA antibody (e). (f). Histograms: image and statistical analyses were performed on five sections of three different mice. TL stain was used as an indication of macrophage infiltration and ssDNA staining as a marker of apoptosis. Panels (ii–vi), representative data obtained from tissue sections from three different brains from either wild‐type (WT) or knockout (KO) animals of comparable clinical scores.
