Figure 3. Role of PSD‐95‐anchored Pyk2 in NMDAR function.
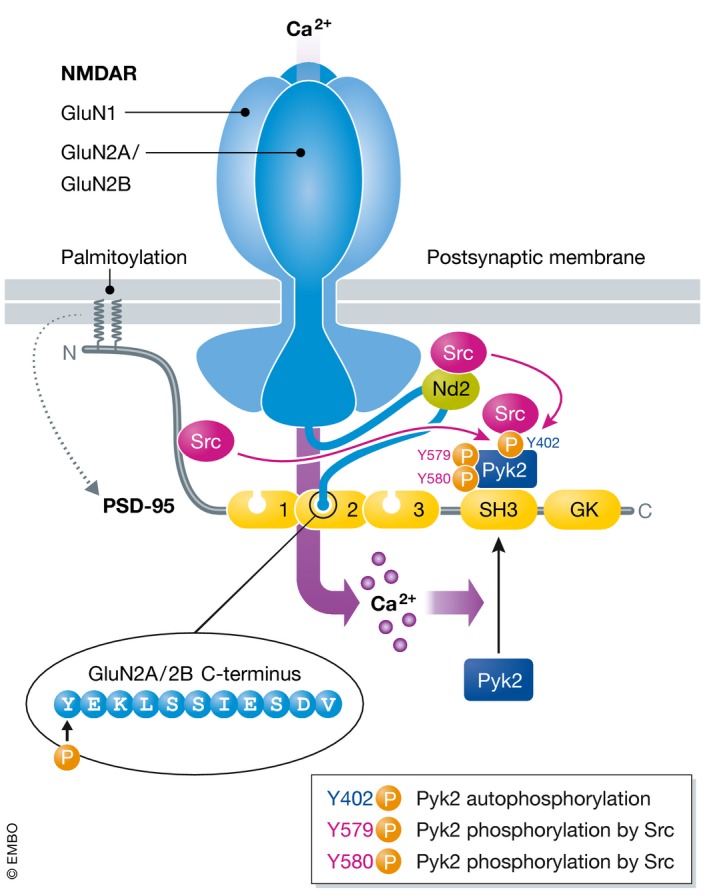
Ca2+ influx induces Pyk2 relocation to postsynaptic sites via Ca2+/CaM‐stimulated binding to the SH3 domain of PSD‐95 (Bartos et al, 2010). At the same time, Pyk2 trans‐autophosphorylates on Y402 (Bartos et al, 2010), which creates a binding site for the SH2 domain of Src. Src might be recruited to pY402 from the N‐terminus of PSD‐95, which binds Src and modestly suppresses Src activity (Kalia et al, 2006), or from NADH dehydrogenase 2 (ND2), a Src‐anchoring protein in the NMDAR complex in addition to PSD‐95 (Gingrich et al, 2004). Binding to pY402 activates Src, which in turn phosphorylates Pyk2 on Y579 and Y580 located in its activation loop to strongly augment Pyk2 activity (Park et al, 2004; Yang et al, 2013). Src binding obviously protects pY402 from dephosphorylation, which is mediated by STEP (Xu et al, 2012). In addition, Y402 phosphorylation can likely be more effectively renewed by Pyk2 when phosphorylated by Src on Y579/Y580 for self‐perpetuating activation of this Pyk2/Src complex as a form of molecular memory (Bartos et al, 2010).
