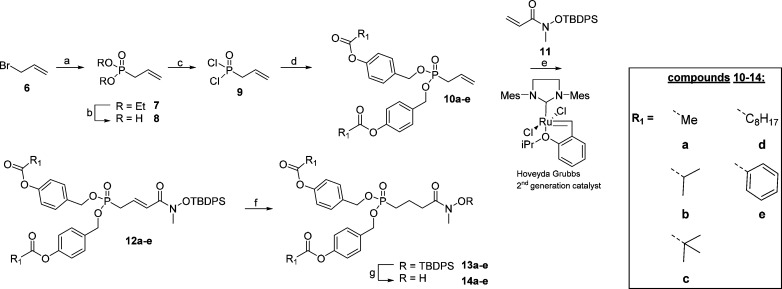
Scheme 1. Synthesis of Acyloxybenzyl Prodrugs.
Reagents and conditions: (a) triethyl phosphite, 150 °C (97%); (b) (i) TMSBr, DCM; (ii) H2O, THF; (c) oxalyl chloride, DMF, DCM, 45 °C; (d) acyloxybenzylalcohol, DIPEA, pyridine, DCM (53–60% over 3 steps); (e) Hoveyda–Grubbs second generation catalyst, toluene, 70 °C (58–77%); (f) NiCl2·6H2O, NaBH4, THF (26–75%); (g) HF·pyridine, pyridine, THF, 0 °C (63–80%).