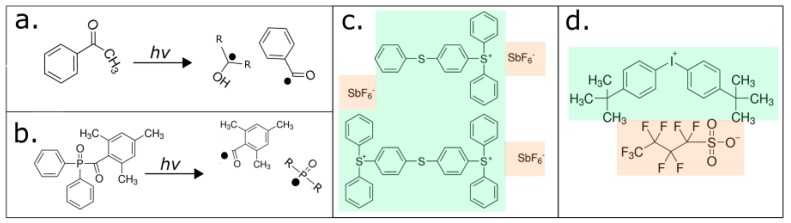
Figure 2.
The two photoinitiator (PI) mechanisms are radical (a,b) and cationic (c,d). In the radical system, absorption of light (hv) produces a free radical by homolytic cleavage, propagating polymerization, for example, as in (a) hydroxyacetophenone and (b) phosphine-oxide, where R represents a methyl group in (a) and a phenyl ring in (b). In the cationic PI system, in PIs such as (c) triarylsulfonium hexafluoroantimonate salts and (d) bis(4-tert-butylphenyl)iodonium perfluoro-1-butanesulfonate, light is absorbed causing heterolytic and homolytic cleavage that forms a cationic portion (labeled in green) and anionic portion (labeled in pink). The generated molecules are reactive with monomers, forming an acid and free radicals, propagating polymerization (open source image adapted from [36]).