Figure 1.
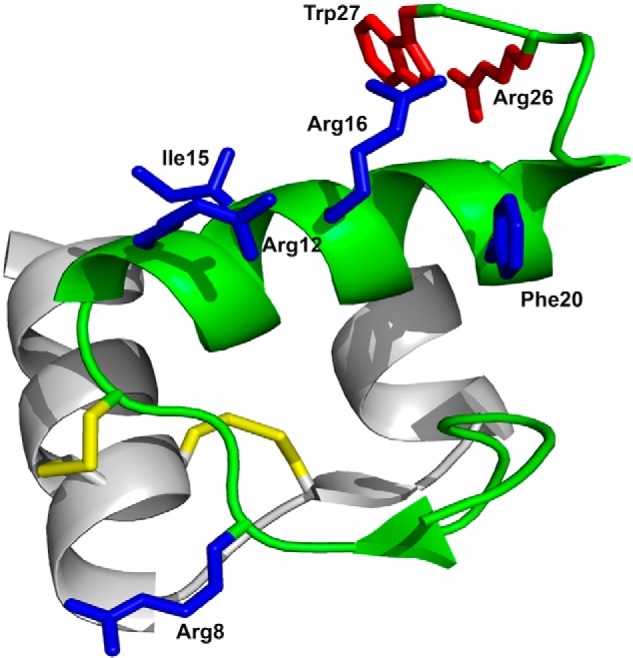
Structure and receptor-interacting amino acid residues of relaxin-3. The A-chain, shown in gray, consists of two antiparallel helices connected by a β-strand. The B-chain, shown in green, comprises a helical segment spanning from Gly11 to Cys22. Disulfides are shown in yellow. Side chains important for binding to RXFP3 and for activating RXFP3 are shown in blue and red, respectively. The R3 B1-22R antagonist retains only the B-chain with the two cysteines at positions 10 and 22 replaced with Ser and the C-terminal five amino acid residues 23–27 replaced with a nonnative Arg23.
