Figure 6.
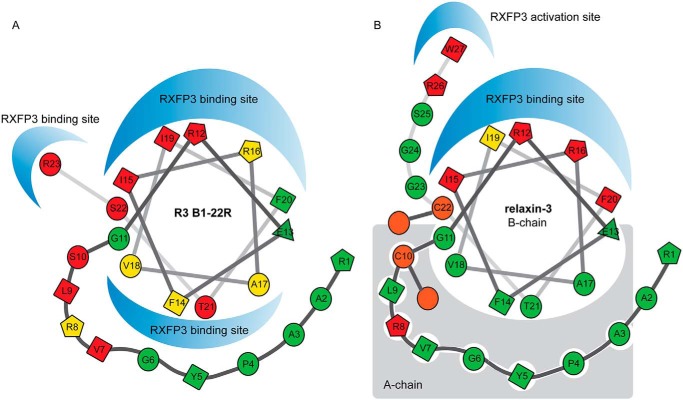
Differences in binding mode of R3 B1-22R and relaxin-3. Structural modifications in the form of staples, Aib substitutions, and Pro substitutions strongly suggest that R3 B1-22R adopts a native-like helical conformation when interacting with RXFP3. Important amino acid residues that are buried and involved in intramolecular interactions in relaxin-3 do contribute to the RXFP3 interactions in R3 B1-22R. We propose that the positioning of the helix differs in R3 B1-22R, creating an additional interaction surface. Positions that showed no tolerance to substitution are shown in red, positions with some tolerance are shown in yellow, and positions where substitutions were widely accepted are shown in green. Positions in relaxin-3 that are not exposed were not probed for binding.