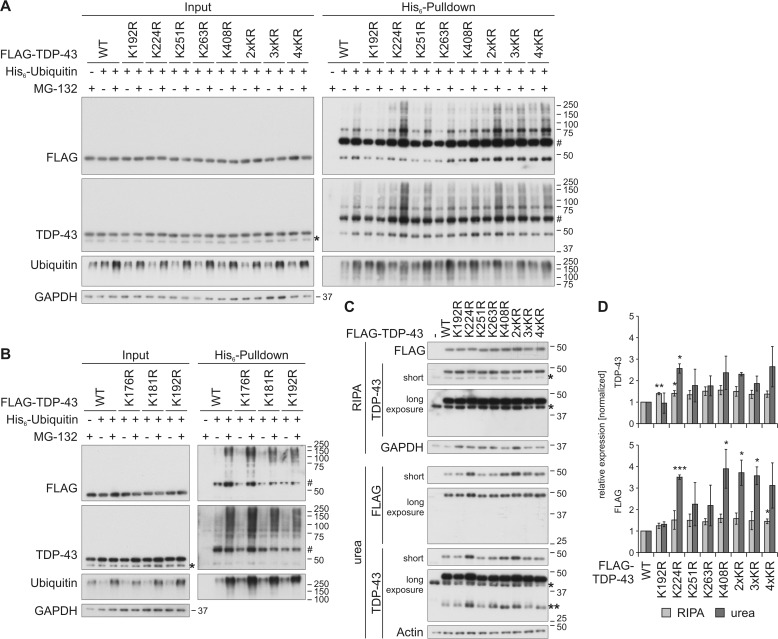
Figure 3.
Characterization of ubiquitinylation of C-terminal lysine residues in FLAG–TDP-43. A and B, HEK293E cells overexpressing His6-ubiquitin (+) or vector control (−), and FLAG–TDP-43WT or indicated lysine mutants for 48 h were treated with MG-132 (+) or DMSO (−) for 2 h. The urea-soluble lysates were prepared, and His6-ubiquitin–conjugated proteins were pulled down from cell lysates. Total-cell lysates (Input) and Ni-NTA–agarose eluates were analyzed by Western blotting and stained for FLAG, TDP-43, ubiquitin, and GAPDH. Asterisks label endogenous TDP-43, and hash marks indicate monoubiquitinylated TDP-43. C, FLAG–TDP-43WT, C-terminal lysine mutants, or control vector (−) were overexpressed in HEK293E cells for 48 h, and RIPA- and urea-soluble lysates were prepared. The lysates were subjected to Western blot analysis, and the blots were stained with antibodies detecting FLAG and TDP-43 and GAPDH and actin as loading controls. Endogenous TDP-43 is labeled with one asterisk; FLAG–TDP-43 derived 35-kDa CTFs are labeled with two asterisks. D, quantification of at least n = 3 experiments is as in C. Band intensities of TDP-43 (upper graph) and FLAG probings (lower graph) were normalized to WT levels. Data represent the mean ± S.D. *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.005.