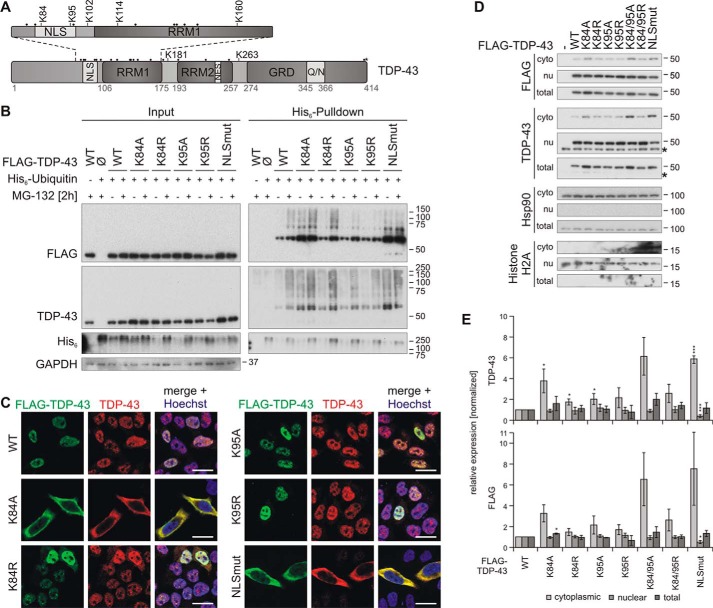
Figure 4.
Characterization of TDP-43 NLS lysine mutant ubiquitinylation and subcellular localization. A, schematic overview of TDP-43 ubiquitinylated lysine (K) residues that were identified by MS. All other lysine residues are indicated with dots. B, His6-ubiquitin (+) or vector control (−) and FLAG–TDP-43WT, NLS lysine mutants, or FLAG control vector (Ø) were overexpressed in HEK293E cells. Proteasomal inhibition with MG-132 for 2 h was followed by cell lysis in urea buffer and affinity purification of His6-ubiquitinylated proteins. Total protein (Input) and Ni-NTA–agarose eluates (His6 Pulldown) were subjected to Western blot analysis and stained with antibodies against FLAG, TDP-43, His6, and GAPDH. C, HEK293E cells overexpressing FLAG–TDP-43WT and NLS lysine mutants were immunolabeled with mouse anti-FLAG (green) and rabbit anti-TDP-43 (red), and cell nuclei were counterstained with Hoechst 33342 (blue). Scale bars are 20 μm. D, nuclear–cytoplasmic fractionation was performed on HEK293E cells that overexpressed FLAG–TDP-43WT or the indicated NLS lysine mutants. Cytoplasmic (cyto), nuclear (nu), and total lysates were analyzed by Western blotting with antibodies detecting FLAG, TDP-43, and Hsp90 as cytoplasmic marker, and histone H2A as nuclear marker. Asterisks label endogenous TDP-43. E, quantification of TDP-43 (upper graph) and FLAG (lower graph) signals from at least n = 3 experiments as in D. Band intensities were normalized to the FLAG–TDP-43WT signal of the respective fraction. Data represent the mean ± S.D. *, p ≤ 0.05; ***, p ≤ 0.005.