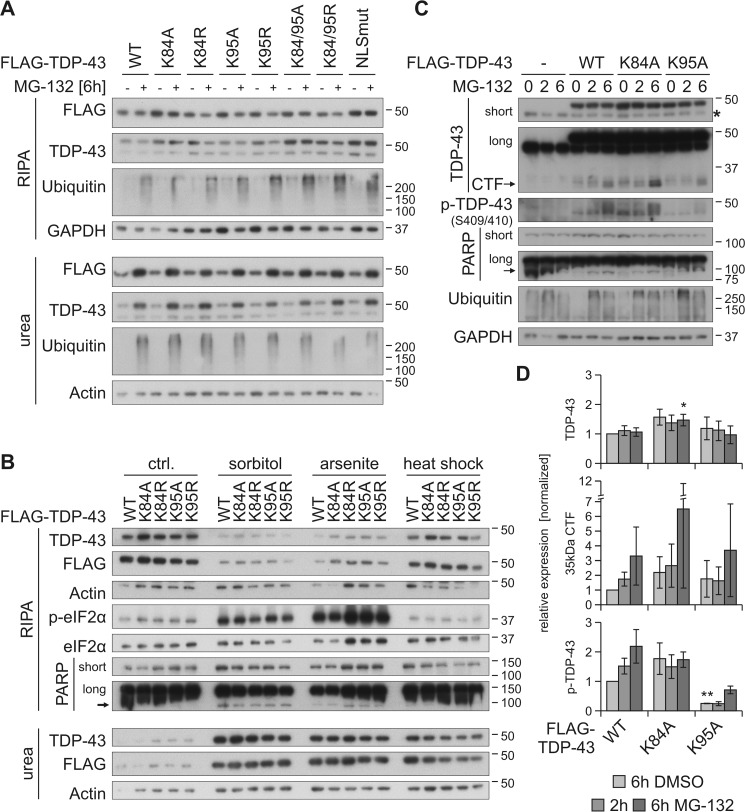
Figure 5.
Prevention of ubiquitinylation at Lys-95 reduces MG-132–stabilized phosphorylation of TDP-43. HEK293E cells were transfected with FLAG–TDP-43WT or NLS lysine mutants and treated with MG-132 (+) or DMSO (−) for 6 h (A) as well as with 0.4 m sorbitol or 200 μm arsenite for 1 h, or subjected to heat shock at 43 °C for 30 min (B). Sequential extraction of the cells was performed, and RIPA and urea lysates were analyzed by Western blotting with antibodies against FLAG, TDP-43, ubiquitin, GAPDH, and actin as loading controls (A). Additional probings of phospho-eIF2α and total eIF2α confirmed stress granule conditions for sorbitol and arsenite treatments and PARP cleavage as a marker of apoptosis (B). C, FLAG–TDP-43WT-, FLAG–TDP-43K84A-, and FLAG–TDP–43K95A-overexpressing HEK293E cells were treated with MG-132 for 2–6 h or DMSO as control (0), followed by lysis with urea buffer. Lysates were subjected to Western blot analysis and stained with antibodies against TDP-43, phospho-TDP-43, PARP, ubiquitin, and GAPDH. D, quantification of FLAG–TDP-43, 35-kDa CTF, and phospho-TDP-43 from at least three experiments as in C. Band intensities were normalized to FLAG–TDP-43WT (6h DMSO), and data represent the mean ± S.D. *, p ≤ 0.05; **, p ≤ 0.01.