Figure 7.
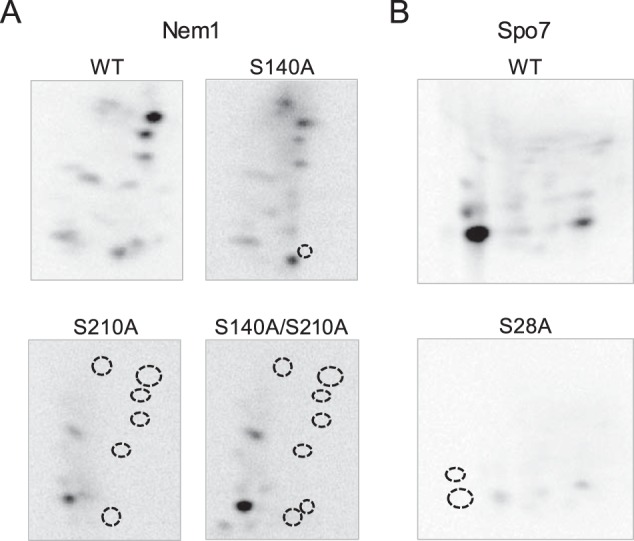
Phosphopeptide-mapping analysis of Nem1 and Spo7 identify their major PKA phosphorylation sites. The WT and indicated mutant forms of Nem1 (A) or the WT and S28A mutant forms of Spo7 (B) were co-expressed in yeast cells and purified by IgG-Sepharose affinity chromatography based on the protein A tag on Nem1. The Nem1–Spo7 complex was incubated with PKA (20 units) and [γ-32P]ATP (100 μm) at 30 °C for 20 min. Following the reaction, samples were subjected to SDS-PAGE followed by transfer to PVDF membrane. The phosphorylated proteins on PVDF membrane were digested with l-1-tosylamido-2-phenylethyl chloromethyl ketone–treated trypsin. The phosphopeptides produced by the proteolytic digestion were separated on cellulose TLC plates by electrophoresis (from left to right) in the first dimension and by chromatography (from bottom to top) in the second dimension. Positions of the phosphopeptides that were absent in the mutant proteins (indicated by the dotted line circles) but present in the WT Nem1 or Spo7 proteins are indicated. The data shown are representative of three experiments.
