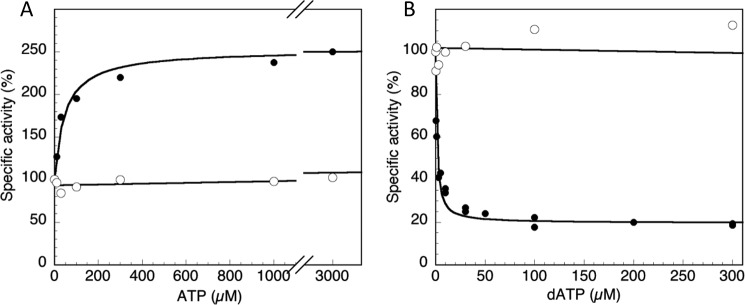
Figure 5.
Inhibition and activation of WT and mutant enzyme activity by dATP and ATP. A and B, ATP titration (A) and dATP titration (B) of enzyme loaded with 2 mm dTTP and GDP as substrate. Specific activities of NrdB proteins were measured with a 10-fold excess of NrdA. WT NrdB (●) had a starting activity of 830 ± 120 nmol/min·mg in the absence of added ATP and reached a Vmax of 1850 ± 200 nmol/min·mg (kcat = 1.8 s−1) in the presence of ATP, whereas NrdBΔ169 (○) had a specific activity of 3400 ± 500 nmol/min·mg (kcat = 2.2 s−1) in the absence of ATP that was not affected by addition of ATP or dATP.