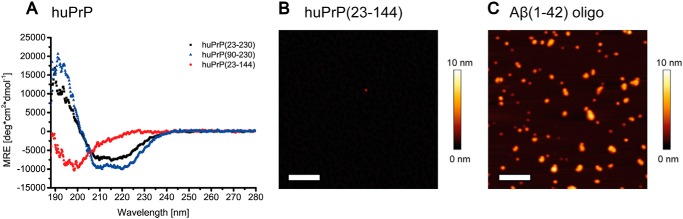
Figure 2.
Analysis of purified huPrP by CD spectroscopy (A) and AFM (B) and of Aβ(1–42)oligo by AFM (C). huPrP(23–230) (A, black) and huPrP(90–230) (A, blue) show predominantly α-helical CD spectra, whereas the N-terminal huPrP(23–144) (A, red) is present in random-coil conformation (MRE, mean residue ellipticity; deg, degrees). Shown are 1-μm2 AFM images of 200 nm huPrP(23–144) (B) or 800 nm Aβ(1–42)oligo (C). Scale bars, 200 nm. huPrP(23–144) forms a thin film on the mica surface, not higher than 1–2 nm (B). The generated Aβ(1–42)oligo species are seen as spherical particles with heights ranging from 1 to 6 nm (C).