Abstract
Aims and Objectives:
The aims of this study were to evaluate the presence of Staphylococcus aureus and Escherichia coli, in polyglycolic acid (PGA) 4-0 and silk sutures, with or without hyaluronic acid (HA) treatment.
Materials and Methods:
This in vitro study measured S. aureus and E. coli growth on PGA and silk sutures, through incubation in agar media for 24 h. The suture length was 10 cm and divided into three parts: A (8 h), B (16 h), and C (24 h), which were observed every 8 h, followed by suspension on a microscopic slide. This was repeated thrice. The number of S. aureus and E. coli cells was recorded and compared between the suture types.
Results:
The mean S. aureus colony forming units (CFUs) differed at each time point between non-HA and HA-PGA sutures (P = 0.0016), with a greater number of CFUs on non-HA-PGA. The mean S. aureus CFUs were significantly higher on non-HA silk than on HA-silk sutures (P = 0.008). There was a significant increase in E. coli CFUs on non-HA silk than on HA-silk sutures (P = 0.008). E. coli CFUs were higher on non-HA-PGA than on HA-PGA sutures (P = 0.006). We performed repeated measures two-way ANOVA (SPSS version 13.0) for comparison between group factors and time points and Posthoc analysis using independent samples t-test.
Conclusions:
HA reduced wicking in both PGA and silk sutures.
KEYWORDS: Escherichia coli, hyaluronic acid impregnated silk, polyglycolic 4-0, Staphylococcus aureus
INTRODUCTION
Sutures are used for different treatment procedures in many surgical specialties. They play a pivotal role in ligating vessels and approximating tissues. In addition, they enhance primary healing and control hemorrhage. Commercially, available sutures can be composed of different materials. The most common options are natural or synthetic, mono or multifilament, and degradation - absorbable or nonabsorbable, which are used for different specialized situations. Furthermore, there are advantages and disadvantages to each type, depending on the nature of the situation in which they are being used.
Periodontal sutures are selected depending on various factors, such as absorbability, ease of handling, strength, and structure. Monofilament sutures have low tie-down resistance and tissue drag and show less infection in surrounding tissues when compared with braided suture materials. In addition, there is less colonization of microorganisms, defined as a “wicking effect.” Conversely, multifilaments are easy to handle and tie because of reduced stiffness. However, they exhibit a higher amount of tissue drag, capillary action, and bacterial harboring than monofilament sutures.[1]
The capillary action of multifilament sutures is due to the interstitial spaces between filaments, and this action acts as a wick, transmitting fluid, and bacteria along the length of the suture material. Therefore, their use is avoided in inflamed or infected tissue. Multifilament sutures can be coated to minimize unwanted capillary action,[2] which reduces bacterial colonization. In this study, we assess silk and polyglycolic acid (PGA) 4-0 sutures. These are multifilament and monofilament sutures, respectively, that are commonly used in periodontal surgical procedures. The strength of PGA reduces significantly over time, but its initial strength is greater than that of silk.[3] In addition, reduced inflammation is observed when using PGA sutures, but silk sutures are more commonly used due to their low-cost availability.
In recent years, chemically modified suture materials have been introduced to reduce the incidence of postsurgical infection and healing time. Hyaluronic acid (HA) has been commonly used for wound closures and healing tissue.[4] Preliminary clinical trials conducted by Pagnacco et al.,[5] revealed the anti-inflammatory, anti-edematous, and antibacterial properties of HA in periodontal disease, which is mainly caused by microorganisms present in subgingival plaque. The highly biocompatible and nonimmunogenic nature of HA has led to its use in a number of clinical applications, including supplementing joint fluid in arthritis; as a surgical aid in eye surgery; and facilitating the healing and regeneration of bone, surgical wounds, and periodontal tissue.
The aim of this study was to assess the effectiveness of HA-treated sutures at reducing bacterial colonization. We compared silk and PGA sutures, which are commonly used in periodontal surgical procedures.
MATERIALS AND METHODS
Necessary approvals were obtained from institutional review board with letter no. REC/28082018.
PREPARATION OF SUTURES
The sutures both PGA and silk were procured from the dental clinics; and each of the sutures was sectioned into 10-cm length to allow adequate room for placement into the petri dish. The petri dish was treated with HA, which was taken from the pharmacy laboratory. This experiment was conducted in the pharmacy laboratory under aseptic conditions. Black silk and PGA sutures were purchased from (Futura Surgicare Pvt. Ltd, India). Sutures were treated with HA using Gengigel® (Ricerfarma S.r.l., Milano, Italy), which contains high-molecular-weight fractions of 0.2% HA in a gel formulation. Gengigel® is used to treat plaque-induced gingivitis as an SRP adjunct.
SUTURE INCUBATION
Silk, HA-silk, PGA, and HA-PGA sutures were placed in trypticase soy agar II with 5% sheep blood (Becton Dickinson, Germany). Each suture type (10 cm) was incubated in triplicate (A, B, and C). A, B, and C samples were observed after 8, 16, and 24 h, respectively, and evaluated for colony forming units (CFUs).
ANALYSIS OF STAPHYLOCOCCUS AUREUS AND ESCHERICHIA COLI COLONY FORMING UNITS
The suture materials, both silk and PGA, were placed in the HA gel for 24 h and then immersed in trypticase soy agar II and 5% sheep blood agar, respectively. Each suture material was sectioned at a length of 10 cm and placed in a test tube for a time frame of 8 h, 16 h, and 24 h, respectively.
STATISTICAL ANALYSIS
Statistical analysis was performed using SPSS version 13.0 (IBM, Armonk, New York, USA). We performed repeated measures two-way ANOVA to compare suture type, treatment (between-group factors), and time points (within-group factor) and assess any interaction. Post hoc analysis was performed using independent samples t-tests between HA and non-HA treated sutures, with Bonferroni correction for multiple comparisons.
RESULTS
The results from our study, based on the ANOVA test between the groups, showed the DF value to be 7 with an F value at 3.629 and with a significance of. 016.
STAPHYLOCOCCUS AUREUS
Relating to silk and HA modified silk, the standard deviation (SD) values were 20 and 15.275, respectively. With PGA and PGA saturated with HA, the SD values were 17.32 and 25.16, respectively [Tables 1 and 3]. This possible difference could be a result of HA not entering into the suture material as the PGA is a monofilament suture material.
Table 1.
Mean Staphylococcus aureus colony forming units with hyaluronic acid-treated and nontreated silk

Table 3.
Mean Staphylococcus aureus colony forming units with hyaluronic acid-treated and nontreated polyglycolic acid 4-0
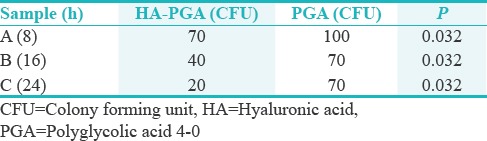
ESCHERICHIA COLI
Relating to silk and HA modified silk, the SD values were 10 and 15.275, respectively. With PGA and HA modified PGA, the SD values were 20 and 25.16, respectively [Tables 2 and 4]. This further validates the view that texture plays an important role in suture microbial retention.
Table 2.
Mean Escherichia coli colony forming units with hyaluronic acid-treated and nontreated silk

Table 4.
Mean Escherichia coli colony forming unit with hyaluronic acid-treated and nontreated polyglycolic acid 4-0

In terms of the significance quotient related to S. aureus and S. aureus with HA-coated silk, the P = 0.008; in S. aureus with PGA and S. aureus coated with HA on PGA, the P = 0.032.
Relating to E. coli with silk and HA-coated silk, the P = 0.008. In terms of E. coli with PGA and E. coli with HA-coated PGA, the P = 0.006.
DISCUSSION
Our study involved the comparison of suture materials for bacterial colonization using HA gel. The sutures were cut in 10-cm length and placed in a petri dish with HA; both the treated and nontreated suture materials were then placed in culture medium trypticase soy agar II with 5% sheep blood (Becton Dickinson, Germany). The samples were evaluated for CFUs at 8, 16, and 24 h, respectively. Our study assessed the effect of HA treatment on bacterial colony forming on two different types of suture, silk, and PGA. We found that HA significantly reduced the number of S. aureus and E. coli CFUs following 8, 16, and 24 h of incubation. These results suggest that treating suture materials with enzymatic solutions, such as HA, significantly reduces the colony forming abilities of bacteria. With regards to our study, HA has shown to reduce the’wicking effect’. There has been a significant decrease in microbial collection,[6] irrespective of the characteristic of the suture material.
When evaluating comparing between microorganisms, in S. aureus, silk and HA-coated silk had a P =0.008 and in S. aureus, PGA and HA coated PGA, the P = 0.032. This difference could be attributed to the structure of the suture material, where silk is a multifilament and PGA is monofilament, which is in agreement with finding of Granet et al.[7] and Qassemyar et al.[8] In relation to E. coli, silk and HA-coated silk had a P = 008 and E. coli, PGA and HA-coated PGA, the P = 006.
Sutures used in oral procedures are continuously bathed in saliva, which contains 7.5 microorganisms/ml × 10 microorganisms/ml. This results in continuous wicking along the suture material at the surgical site, which can cause a prolonged inflammatory reaction. Therefore, many studies have sought to reduce the incidence of infection and inflammation in periodontology.
Grigg et al.[6] have assessed the effects of HA on incision healing in the oral cavity and found that it can accelerate wound healing and reduce inflammation. In addition, our study is in line with Leknes et al.,[9] who assessed the wound healing and anti-inflammatory properties of HA at surgical sites. The role of HA in healing following soft tissue and microsurgeries has been documented in previous studies.[2,10,11] The presence of HA in sutures significantly reduced the number of CFUs in our study. This result has also been shown by Moser et al.[12] In addition, the reduction in CFUs was directly proportional to time, which is in agreement with earlier studies.[13,14]
Silk is a multifilament material and shows more wicking than PGA.[15] The role of various suture materials in wound healing has been documented. They can contribute to inflammatory reactions and differ between patients.[10,16] Previous studies have investigated the wicking effect of silk[7,17] and show similar results to our study. Granet et al.[7] and Qassemyar and Gianfermi[8] have shown the reaction of different tissues and the adherence of bacteria to different suture materials.[18] Although our study was conducted in an in vitro environment and faced challenges with incorporating an enzyme into a suture material, it has given significant results in terms of bacterial colonization. In our study, for both S. aureus and E. coli, HA-modified suture related to both silk and PGA has shown significant results in terms of bacterial colonization.
The colonization of various microbes on different suture materials from different patients has been investigated previously.[19] The results of this study showed that a large number of bacteria colonized silk sutures when compared with PGA. PGA is a monofilament suture; therefore, the interstitial spaces between the PGA filaments are not wide enough to attract bacteria.[12] The application of HA on the suture materials has an antibacterial action on gingival tissues.[3,4,20] HA application to gingival application in cases where mild-to-moderate gingivitis can reduce its incidence to normal or near normal.[1] The role of interstitial spaces between suture materials, especially among braided materials like silk also is a factor to harbor microorganisms.[19] When monofilament suture materials like PGA are taken, the interstitial spaces are almost negligible, this further reduces the grouping of bacteria and reduces considerably when chemically modified, thereby reducing the “wicking effect.”
Further studies may be necessary to compare and combine different anti-wicking methods to further reduce the incidence of inflammation at the site of infection. The need to consider the principles of local delivery of drugs and to incorporate similar technology;[21] in suture, material might give promising results. Furthermore, research in the role of immune mechanisms needs to be considered as it can give better information about the role of specific suture materials in different conditions.[22] In addition, this experiment has been performed with specific suture materials in a controlled environment; therefore, the full effect of wicking could not be established. The results of this study further do not give much importance to the structure of the suture material, silk being multifilament and PGA being monofilament, this factor could be a important parameter to be considered for evaluation in future research methodologies. Time factors also need to be considered, as the sutures need to be assessed for longer duration. Future studies should test different suture materials and incubate them for a longer duration to assess the impact of HA treatment, and offer greater understanding of the physical and biologic properties of wicking between suture materials.
CONCLUSIONS
This experiment provides further evidence of the anti-wicking properties of HA and proposes a novel measure of directly treating sutures with HA to prevent bacterial colonization. Further studies need to be done using different types of suture materials and for longer duration to justify quality and assurance in relation to postsurgical healing.
FINANCIAL SUPPORT AND SPONSORSHIP
Nil.
CONFLICTS OF INTEREST
There are no conflicts of interest.
REFERENCES
- 1.Obermeier A, Schneider J, Harrasser N, Tübel J, Mühlhofer H, Pförringer D, et al. Viable adhered Staphylococcus aureus highly reduced on novel antimicrobial sutures using chlorhexidine and octenidine to avoid surgical site infection (SSI) PLoS One. 2018;13:e0190912. doi: 10.1371/journal.pone.0190912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Obermeier A, Schneider J, Föhr P, Wehner S, Kühn KD, Stemberger A, et al. In vitro evaluation of novel antimicrobial coatings for surgical sutures using octenidine. BMC Microbiol. 2015;15:186. doi: 10.1186/s12866-015-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varma S, Abufanas S, Ali M, Nadhim N, Khan M, Abuhijleh E. Comparison of wicking effect of different sutures: An in vitro study. Int J Curr Res. 2017;9:61469–72. [Google Scholar]
- 4.Galli F, Zuffetti F, Capelli M, Fumagalli L, Parenti A, Testori T, et al. Hyaluronic acid to improve healing of surgical incisions in the oral cavity: A pilot multicentre placebo-controlled randomised clinical trial. Eur J Oral Implantol. 2008;1:199–206. [PubMed] [Google Scholar]
- 5.Pagnacco A, Vangelisti R, Erra C, Poma A. Double-blind clinical trial versus placebo of a new sodium-hyaluronate-based gingival gel. Attual Ter Int. 1997;15:1–7. [Google Scholar]
- 6.Grigg TR, Liewehr FR, Patton WR, Buxton TB, McPherson JC. Effect of the wicking behavior of multifilament sutures. J Endod. 2004;30:649–52. doi: 10.1097/01.don.0000121617.67923.05. [DOI] [PubMed] [Google Scholar]
- 7.Granet DB, Hertle RW, Ziylan S. The use of hyaluronic acid during adjustable suture surgery. J Pediatr Ophthalmol Strabismus. 1994;31:287–9. doi: 10.3928/0191-3913-19940901-04. [DOI] [PubMed] [Google Scholar]
- 8.Qassemyar Q, Gianfermi M. Supermicrosurgery and hyaluronic acid: Experimental feasability study of a new method. Ann Chir Plast Esthet. 2015;60:e59–65. doi: 10.1016/j.anplas.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 9.Leknes KN, Selvig KA, Bøe OE, Wikesjö UM. Tissue reactions to sutures in the presence and absence of anti-infective therapy. J Clin Periodontol. 2005;32:130–8. doi: 10.1111/j.1600-051X.2005.00647.x. [DOI] [PubMed] [Google Scholar]
- 10.Agubata CO, Okereke C, Nzekwe IT, Onoja RI, Obitte NC. Development and evaluation of wound healing hydrogels based on a quinolone, hydroxypropyl methylcellulose and biodegradable microfibres. Eur J Pharm Sci. 2016;89:1–10. doi: 10.1016/j.ejps.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 11.Pirnazar P, Wolinsky L, Nachnani S, Haake S, Pilloni A, Bernard GW, et al. Bacteriostatic effects of hyaluronic acid. J Periodontol. 1999;70:370–4. doi: 10.1902/jop.1999.70.4.370. [DOI] [PubMed] [Google Scholar]
- 12.Moser JB, Lautenschlager EP, Horbal BJ. Mechanical properties of polyglycolic acid sutures in oral surgery. J Dent Res. 1974;53:804–8. doi: 10.1177/00220345740530040601. [DOI] [PubMed] [Google Scholar]
- 13.Okamoto T, Rosini KS, Miyahara GI, Gabrielli MF. Healing process of the gingival mucosa and dental alveolus following tooth extraction and suture with polyglycolic acid and polyglactin 910 threads. Comparative histomorphologic study in rats. Braz Dent J. 1994;5:35–43. [PubMed] [Google Scholar]
- 14.Masood R, Hussain T, Umar M, Azeemullah A, Areeb T, Riaz S, et al. In situ development and application of natural coatings on non-absorbable sutures to reduce incision site infections. J Wound Care. 2017;26:115–20. doi: 10.12968/jowc.2017.26.3.115. [DOI] [PubMed] [Google Scholar]
- 15.Katz S, Izhar M, Mirelman D. Bacterial adherence to surgical sutures. A possible factor in suture induced infection. Ann Surg. 1981;194:35–41. doi: 10.1097/00000658-198107000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sethi KS, Karde PA, Joshi CP. Comparative evaluation of sutures coated with triclosan and chlorhexidine for oral biofilm inhibition potential and antimicrobial activity against periodontal pathogens: An in vitro study. Indian J Dent Res. 2016;27:535–9. doi: 10.4103/0970-9290.195644. [DOI] [PubMed] [Google Scholar]
- 17.Bucci M, Borgonovo A, Bianchi A, Zanellato A, Re D. Microbiological analysis of bacterial plaque on three different threads in oral surgery. Minerva Stomatol. 2017;66:28–34. doi: 10.23736/S0926-4970.16.03966-7. [DOI] [PubMed] [Google Scholar]
- 18.Kumar R, Srinivas M, Pai J, Suragimath G, Prasad K, Polepalle T, et al. Efficacy of hyaluronic acid (hyaluronan) in root coverage procedures as an adjunct to coronally advanced flap in millers class I recession: A clinical study. J Indian Soc Periodontol. 2014;18:746–50. doi: 10.4103/0972-124X.147411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vedamurthy M. Soft tissue augmentation – Use of hyaluronic acid as dermal filler. Indian J Dermatol Venereol Leprol. 2004;70:383–7. [PubMed] [Google Scholar]
- 20.Park JH, Park EJ, Yi HS. Wound healing and anti-inflammatory effects of topical hyaluronic acid injection in surgical-site infection caused by Staphylococcus aureus. Int J Low Extrem Wounds. 2017;16:202–7. doi: 10.1177/1534734617714142. [DOI] [PubMed] [Google Scholar]
- 21.Lee EJ, Huh BK, Kim SN, Lee JY, Park CG, Mikos AG, et al. Application of materials as medical devices with localized drug delivery capabilities for enhanced wound repair. Prog Mater Sci. 2017;89:392–410. doi: 10.1016/j.pmatsci.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lock AM, Gao R, Naot D, Coleman B, Cornish J, Musson DS, et al. Induction of immune gene expression and inflammatory mediator release by commonly used surgical suture materials: An experimental in vitro study. Patient Saf Surg. 2017;11:16. doi: 10.1186/s13037-017-0132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


