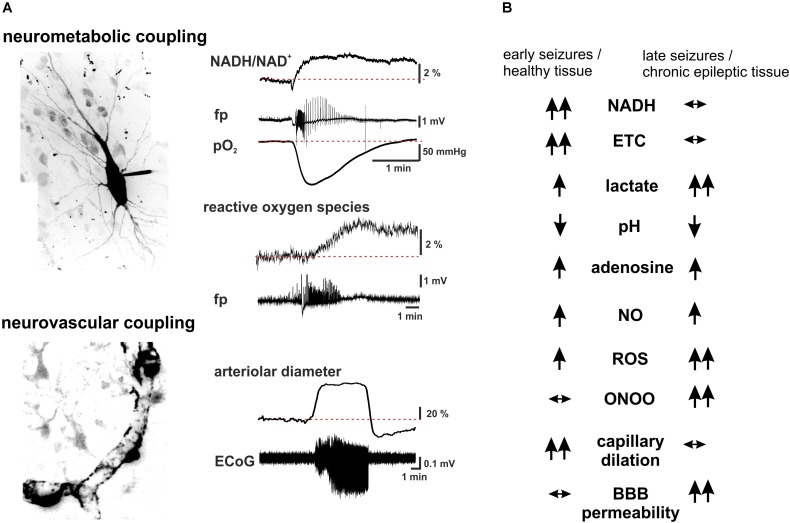
FIGURE 2.
Summary scheme of seizure-associated changes in cellular energy metabolism and cerebral blood. (A) Seizure-associated changes in transmembrane ion gradients ultimately activate neuronal energy metabolism in order to counterbalance the ATP-need of the ion pump activity. In brain slices kept under constant carbogen gassing conditions seizure-associated enhancement of respiration can be measured as a drop in pO2 whereas a shift in the fluorescence of NADH represents the metabolism-dependent redox shift of this electron carrier (fp: local field potential). Whereas the respiration slowly returns to the pre-seizure values following cessation of the activity, the reducing shift in the NADH/NAD+ ratio outlast the changes in respiration. This mismatch might favor the late enhancement of reactive oxygen species (ROS) formation, as represented here by the oxidation of the mitochondrially targeted ethidium derivative, MitoSox in the same preparation. Seizures are associated with reversible arteriolar and capillary vasodilation, the latter likely mediated by contractile pericytes, and a consequent overshooting pO2 response. (B) There are considerable differences in the seizure-associated neurometabolic and neurovascular responses between early and late events during status epilepticus, as well as in healthy and chronic epileptic tissue (arrows indicate the direction of the change of individual parameters). Seizure-associated overshooting NADH reduction eroded during recurrent seizures in vitro and it was almost absent in chronic epileptic tissue from epilepsy patients, due to the free radical dependent damage of mitochondrial dehydrogenases. On the other hand, mitochondrial injury might in turn enhance subsequent formation of reactive oxygen species (ROS) in a vicious cycle. Seizures resulted in lactate release both in control and chronic epileptic tissue samples, whereas in the latter even interictal lactate levels were increased likely reflecting altered expression pattern of monocarboxylate transporters and energy metabolism of glial cells. Seizure-associated enhancement of respiration and lactate release result in a metabolic acidosis, which – along with the enhanced adenosine levels- represents a strong anticonvulsant mechanisms even in chronic epileptic tissue. Despite the induction of massive CBF increase by seizures vascular responsiveness was found to be decreased following status epilepticus, and changes in pericytic reactivity are observable already few minutes after seizure onset. This could be brought by oxidative stress of these cells as described in the no reflow phenomenon after hypoxia-reperfusion. Although seizure-associated increase in nitric oxide (NO) formation contributes to the hyperemia, in the presence of superoxide NO is turned into peroxynitrite with deleterious consequences on microcirculation. Postictal hyporesponsiveness of the microvasculature has been speculated to underlie cognitive disturbances and epilepsia comorbidities, whereas damage of the blood-brain barrier (BBB), albumin leakage and the subsequent transformation of the nervous tissue were shown to be pro- epileptogenic. (hippocampal pyramidal cell and capillary pericytes are just for illustration purposes, some of the traces in A are adopted from Malinska et al., 2010).