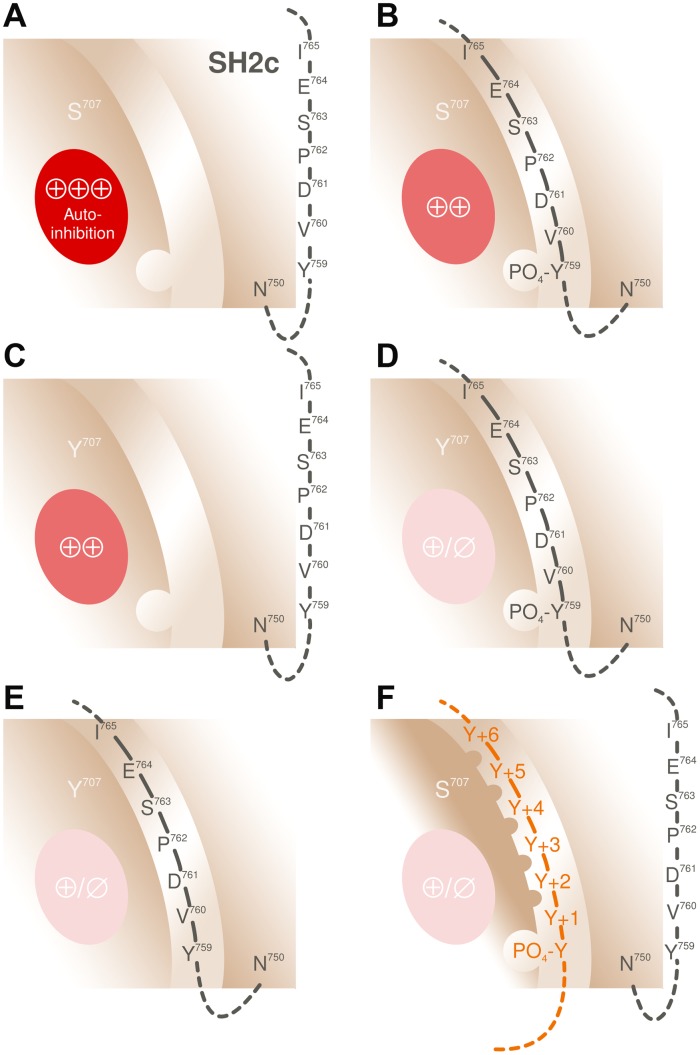
Figure 15. Mechanisms potentially involved in mediating activation of PLCγ2 through mutations in position S707.
(A) In wild-type PLCγ2, the SH2c-SH3 linker peptide carrying the protein tyrosine kinase substrate Y759, does not occupy the pY-peptide-binding groove on cSH2, thus allowing for maximal autoinhibition of PLCγ2 activity by this domain (⊕⊕⊕). (B) Phosphorylation of Y759 causes an interaction of the linker peptide with its binding groove and a reduction of enzyme autoinhibition by cSH2 (⊕⊕), hence, activation of PLCγ2. (C) The activating mutations in position 707, e.g. S707Y, could cause a loss of the autoinhibitory properties of cSH2, thus obviating, at least in part, the requirement of its interaction with the pY759 peptide for enzyme activation. (D) Interaction of the phosphorylated linker peptide could further reduce PLCγ2 autoinhibition. In addition, the structural change induced by the S707 mutations could increase the affinity of the extended cSH2 groove for the pY759 peptide and thus enhance the likelihood of pY-peptide-groove-complex formation. Alternatively, the S707 mutations could alter the overall structure of the pY-peptide-groove-complex even without altering the pY-peptide-groove-interaction, such that the complex attains an even lower affinity for the electronegative counterpart on the catalytic core or a lower (residual) efficacy as an enzyme autoinhibitor (⊕/∅). Consistent with this notion, structural differences have been observed between the pY-peptide-complexed and -uncomplexed forms of the Src SH2 domain, with largest shifts seen for the EF loop residues corresponding to G705, T706, and S707 of PLCγ2 [48] (E) Since the PLCγ1 Y783 peptide has been shown to interact with the peptide-binding groove on PLCγ1 cSH2 even in the absence of Y783 phosphorylation [20], the corresponding mutations at S707 of PLCγ2 could enhance the binding and, hence, the functional effects of even the non-phosphorylated Y759 linker peptide. (F) Alteration of the pY-peptide binding specificity of PLCγ2. It is well known that differences between the EF loops of other SH2 domains are important determinants of the specificity of the pY-peptide-extended-groove binding. For example, Grb2-like SH2 domains contain a bulky tryptophan residue at a position corresponding to G705 of PLCγ2, which protrudes from the EF loop and blocks the pY+3 position, if the phosphopeptide is in an extended conformation. This forces the bound peptides to make a β-turn, which is accomplished by selecting an asparagine residue in the pY+2 position [49, 50]. Replacement of the serine residue corresponding to G705 of PLCγ2 in the Src SH2 domain by a tryptophan residue switched its selectivity for pY-peptides to resemble that of the Sem5/drk/Grb2 SH2 domain [51]. Pertinent to this issue, PLCγ cSH2 domains have been shown to interact with many different peptide ligands, however, they do so with a defined specificity [52–54]. Structural changes at and/or near residue S707 of PLCγ2 may, therefore, alter the specificity of peptide recognition and, hence, the “language” of this domain [55]. The observation that a PLCγ2 mutant carrying mutations expected to block activation by phosphorylation of intramolecular tyrosine residues (4F) and Rac (F897Q) was still activated by EGF is consistent with this view (cf. Figure 11).