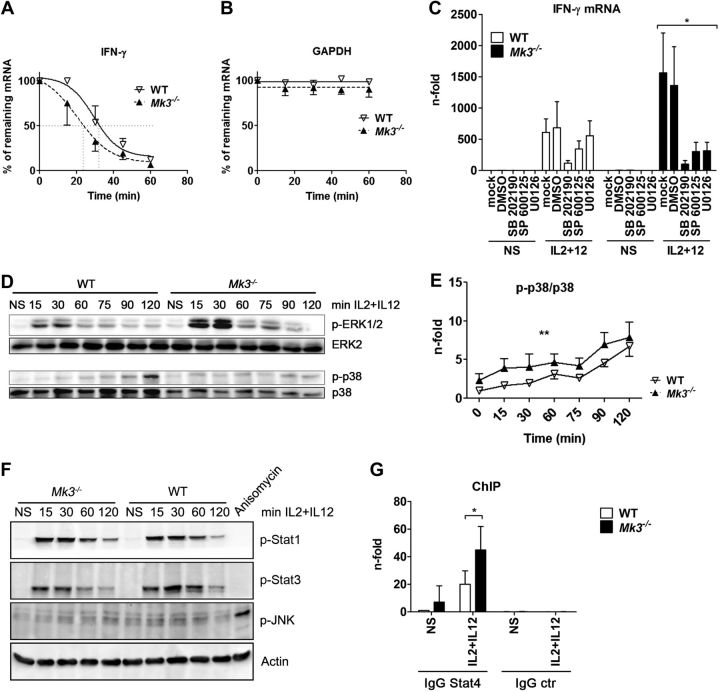
Figure 2.
Mk3 deficiency leads to enhanced activation of ERK1/2 and p38 and induces a stronger binding of Stat4 to the IFN-γ promoter, but does not alter IFN-γ mRNA stability. A, B) NK cells from WT and Mk3−/− mice were stimulated with IL-2 + IL-12 for 3 h and treated with actinomycin D at the indicated time points. Amounts of IFN-γ (A) and GAPDH (B) mRNA were determined by qRT-PCR (N=3, with NK cells pooled from 3 animals in each experiment). Statistical analysis, 2-way ANOVA. C) NK cells isolated from C57Bl/6 WT and Mk3−/− mice were preincubated for 30 min with either DMSO or the indicated inhibitor and stimulated for 3 h with IL-2 + IL-12. IFN-γ mRNA was analyzed by qRT-PCR (N=3–4, with NK cells pooled from 3 animals in each experiment). *P < 0.05, Kruskal-Wallis test. D) NK cells were isolated from C57Bl/6 WT and Mk3−/− mice, cultured, and stimulated for the indicated times with IL-2 + IL-12 and subsequently analyzed for the activation of ERK1/2 and p38 by Western blot (N=3). E) p38 data from D were quantified with AIDA software (Raytest) **P < 0.01; 2-way ANOVA (N=3). F) Isolated NK cells from C57Bl/6 WT or Mk3−/− mice were stimulated with IL-2 + IL-12 for the indicated times, and the cell lysates were subsequently analyzed by Western blot for activation of JNK, Stat1, and Stat3. G) ChIP assays were performed on isolated NK cells of C57Bl/6 WT and Mk3−/− mice using Stat4 antibody or an IgG control antibody. Coprecipitated IFN-γ promoter DNA was analyzed with qRT-PCR and compared to the whole amount of the IFN-γ promoter DNA in the samples. DNA amount bound to Stat4 in unstimulated WT cells was arbitrarily set at 1 (N=4–5, with cells pooled from 3 animals in each experiment). *P < 0.05; Mann-Whitney test.