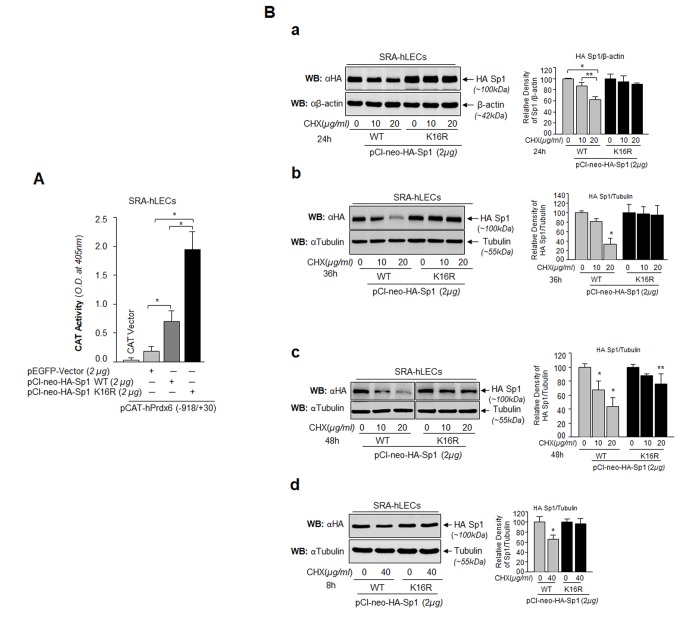
Figure 9.
Sp1 (K16R) mutated at Sumoylation site enhanced its transcription potential by increasing steady state of Sp1 in cells. (A) SRA-hLECs were cotransfected with wild type Prdx6 promoter linked to CAT along with either pCl-neo-HA-Sp1 or pCl-neo-HA-Sp1K16R as shown. After 72h cell lysates were analyzed for CAT activity. Histograms represent values derived from three independent experiments. *p < 0.001. (B) Relative protein stability of Sp1WT vs Sumoylation-deficient mutant Sp1K16R. SRA-hLECs were transiently transfected with pCl-neo-HA-Sp1WT or its mutant, pCl-neo-HA-Sp1K16R. After 36h, the transfectants were treated with different concentrations of CHX (10 and 20µg/ml) for 24h (Ba) or CHX (10 and 20µg/ml) for 36h (Bb) or CHX (10 and 20µg/ml) for 48h (Bc) or CHX (40µg/ml) for 8h (Bd) or as indicated. Total lysates with equal amounts of proteins were western blotted (WB) with anti-HA antibody. Anti-β-actin or anti-Tubulin antibodies were used as loading control. The percentage of remaining Sp1 (Sp1WT and its mutant Sp1 K16R) protein after the CHX translational inhibitor treatment is presented as histogram in right side of Western blot based upon densitometry quantitation. Control vehicle (DMSO) vs CHX treated, **p<0.05; *p<0.001.