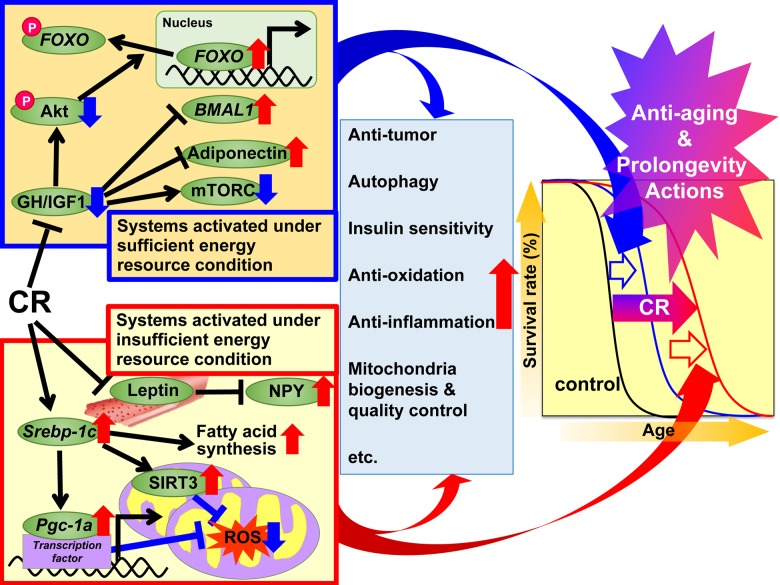
Figure 1.
Proposed mechanisms of the anti-aging and prolongevity actions of caloric restriction (CR) based on the adaptive response hypothesis. On the basis of the adaptive response hypothesis of CR, we propose that the regulatory mechanisms of CR are classified into two systems, which additively extend lifespan. The first system is activated under sufficient energy resource conditions, when there is grace for free use of energy, and animals grow well, reproduce more, and store excess energy as triglyceride (TG) in white adipose tissue (WAT) for later use, but not to such an extent that they become obese. This system involves growth hormone (GH)/insulin-like growth factor 1 (IGF1), Akt, forkhead box O (FOXO), mechanistic target of rapamycin complex (mTORC), adiponectin and brain and muscle aryl like protein 1 (BMAL1) signaling. In CR animals, these signals act to suppress anabolic reactions. The second system is activated under insufficient energy resource conditions, when there is no grace for free use of energy, and animals suppress growth and reproduction and shift energy use from growth and reproduction to maintenance of biological function, but not to such an extent that they become severely starved. This system involves sterol regulatory element-binding protein 1c (SREBP-1c), sirtuin (SIRT), peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α), mitochondrial reactive oxygen species (ROS), leptin and neuropeptide Y (NPY) signaling. In CR animals, these signals act to use energy effectively. Moreover, various signals and/or factors might contribute to CR-associated beneficial actions including anti-oxidative, anti-inflammatory, anti-tumor and other CR actions to a different extent in each tissue or organ, and thereby lead to anti-aging and prolongevity.