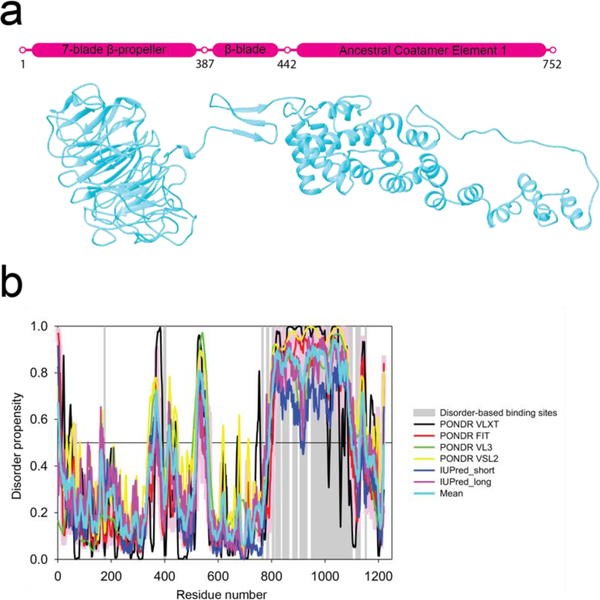
Figure 1.
Sequence analysis of Sec31. a) Schematic diagram and flexibly fitted model from crystal structures of Sec31 domains (ΔC half-edge model derived from wt map) (Noble et al., 2012). Marked residues 387–442 contain the Sec13 binding β-sheet and the two coil regions connecting it to the N-terminal β-propeller domain and the C-terminal ACE1 domain. The latter coil region is also known as the hinge which enables the flexing of the ACE1 domain (Fig 5e). Residues 752–1273 (not shown) are known to constitute a disordered region for which there is no crystal structure or electron-microscopy map which is followed by a small α-helical domain. b) Intrinsic disorder propensity analysis of human Sec31A sequence by a set of commonly used disorder predictors, PONDR® VLXT, PONDR® VL3, PONDR® VSL2, IUPred_long and IUPred_short. Mean disorder propensity represent averaged disorder scores per residue from six individual predictors (indicated in the figure legend). All the scores consistently indicate a high disorder propensity (above 0.5) for the C-terminal region except for the small α-helical domain. Positions of potential disorder-based binding sites found by ANCHOR are shown by gray shaded areas.