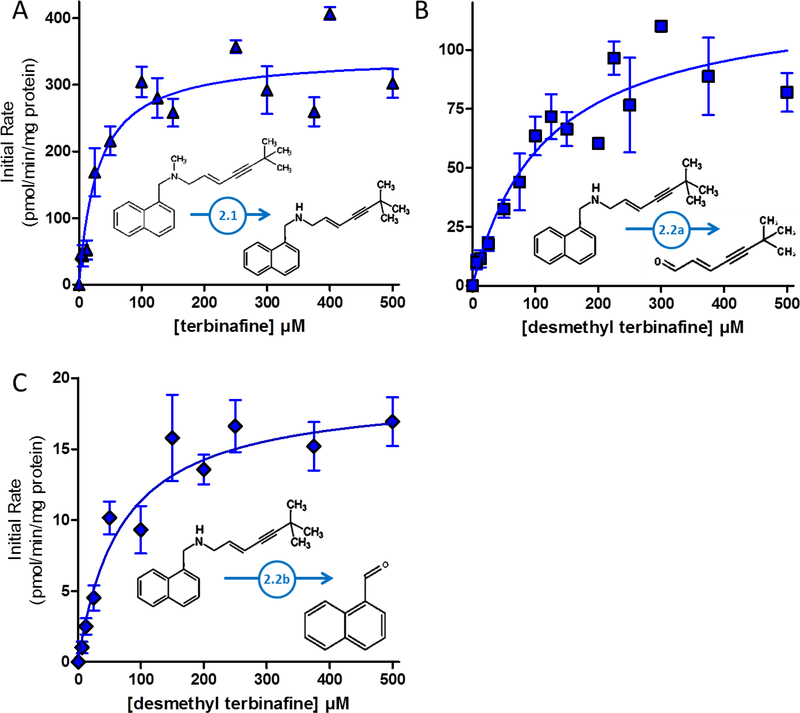
Fig. 4. Steady-state kinetic profiles for Pathway 2 of terbinafine N-dealkylation.
N-Dealkylation of terbinafine and desmethyl-terbinafine yielded metabolic kinetics for three metabolites derived from Pathway 2 as illustrated in Fig. 1. The kinetic profiles include those for (A) desmethyl-terbinafine (dansyl chloride labeled) from terbinafine, (B) TBF-A (dansyl hydrazine labeled) from desmethyl-terbinafine (Path 2.2A), and (C) naphthaldehyde (dansyl hydrazine labeled) from desmethyl-terbinafine (Path 2.2B). All sets of data were fit best to the Michaelis-Menten equation (p < 0.05), and the corresponding constants reported in Table 1. Nine experimental reactions were carried out with terbinafine and desmethyl-terbinafine as substrate. Reaction conditions and data analysis were carried out as described in Materials and Methods.