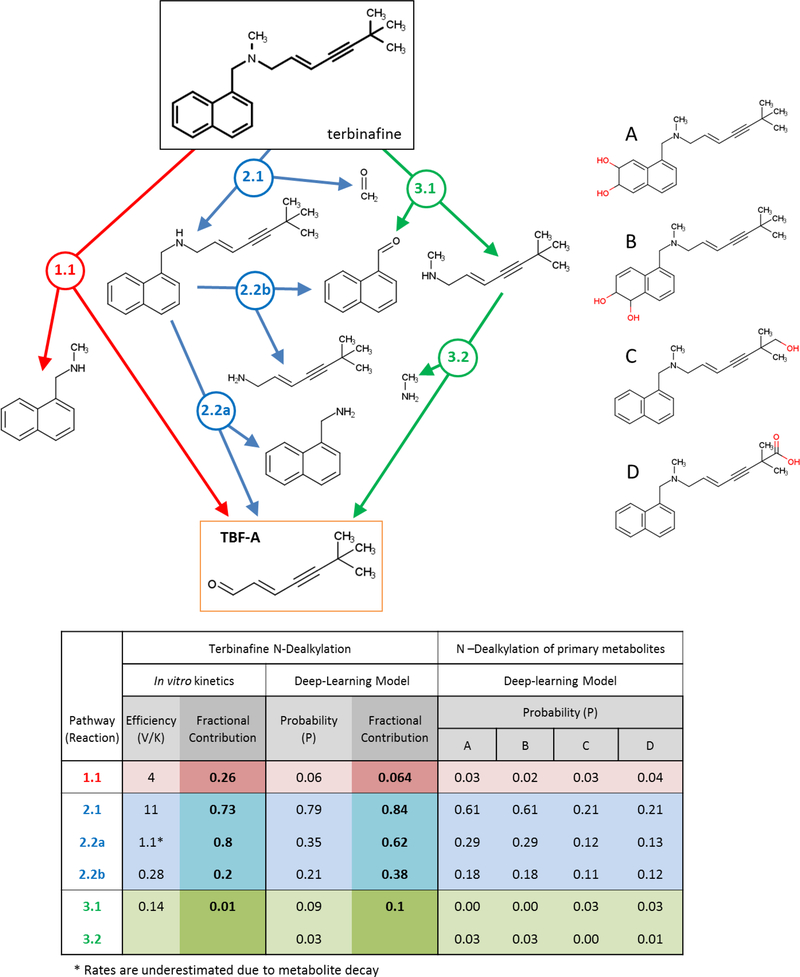
Fig. 6. An overview of the kinetic efficiencies, model predictions and fractional contributions for terbinafine N-dealkylation pathways.
Reactions are labeled by pathway number, reaction number, and branch designation if applicable (e.g. 2.2a). The table lists information gained from experimental and computational modeling studies as well as their limitations (see Results for details). These data include the experimentally measured Vmax/Km values, modeled reaction probabilities, and fractional contributions for each reaction at the pathway branching point based on the respective data sets. The model was also used to predict probabilities for N-dealkylations of known primary metabolites of terbinafine, for which we have no experimental kinetics. *TBF-A efficiencies are underestimated due to decay kinetics competing with those for formation of TBF-A.