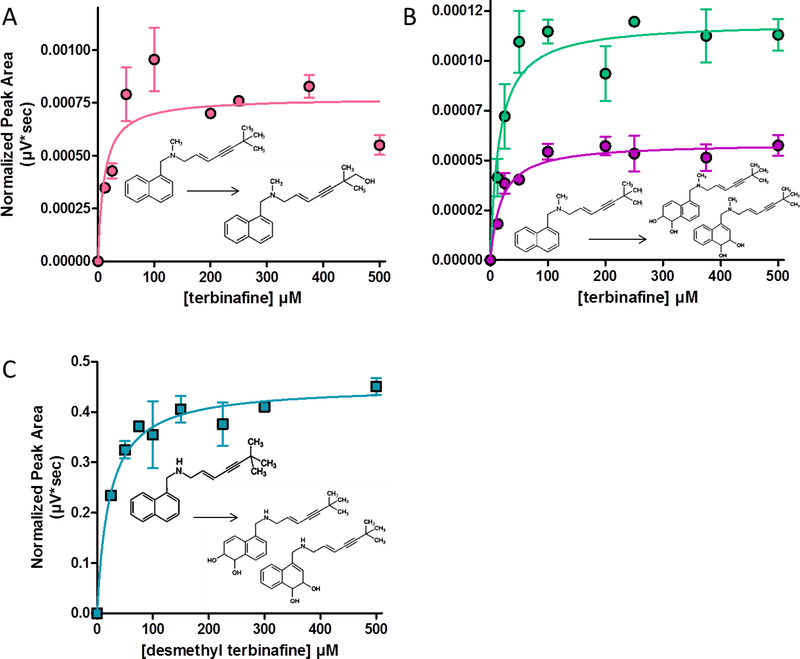
Fig. 7. Steady-state kinetic profiles for metabolites of other oxidation pathways of terbinafine metabolism.
Steady-state reactions yielded several metabolites from oxidative pathways not involving N-dealkylation. No quantitative standards for the products were available, so data was reported based on MS peak area. No labeling was used for detection of these metabolites. Terbinafine reactions yielded profiles for (A) hydroxyterbinafine and (B) two isomers of terbinafine dihydrodiol (m/z). (C) Desmethyl-terbinafine reactions yielded profiles for two isomers of desmethyl-terbinafine dihydrodiol, (m/z 312) not shown in Fig. 1. Three experimental reactions were carried out for each substrate. Reaction conditions were carried out as described in Materials and Methods.