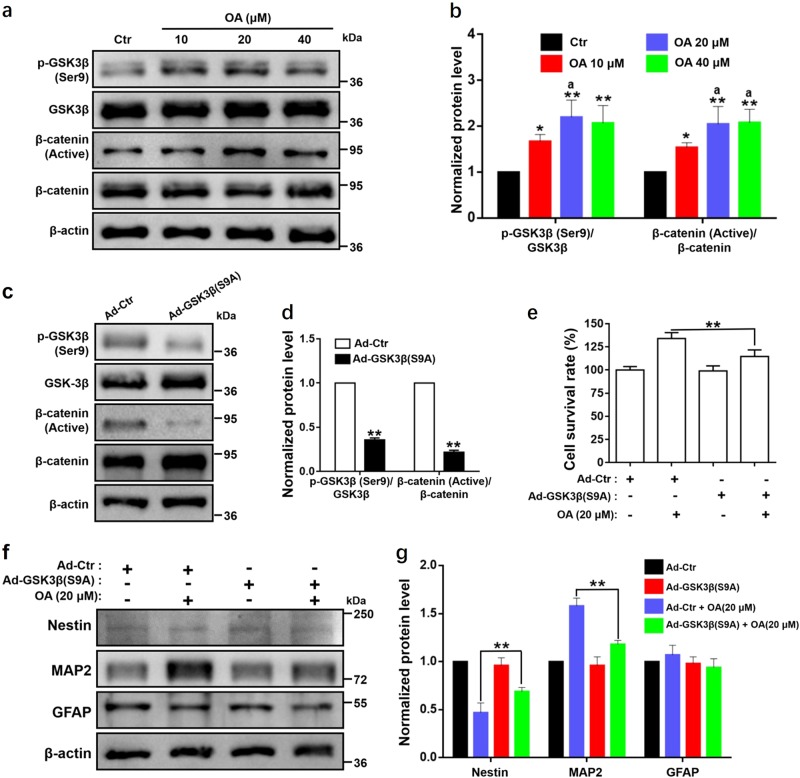
Fig. 5. OA regulates the activation of GSK3β in NSC induction.
Proliferation and differentiation of NSCs mediated by OA depends on the suppressing of the activation of GSK3β. a Western blot analyses and b relative optical density of diverse pathway markers including β-catenin (active), β-catenin, p-GSK3β (Ser9), and GSK3β in NSCs after treatment with OA for 7 days. *p < 0.05 and **p < 0.01, compared with the Ctr group; ap < 0.05, compared with 10 μM OA group. c Western blot analyses and d relative optical density of p-GSK3β (Ser9), GSK3β, p-GSK3β (Ser9), and GSK3β in NSCs after transfection with GSK3β (S9A) adenovirus for 2 days. **p < 0.01, compared with the Ad-Ctr group. e NSCs with GSK3β (S9A) significantly suppressed cell viability, as examined by CCK-8 assay. **p < 0.01, compared with Ad-Ctr + 20 μM OA group. f Western blot analyses and g relative optical density of diverse differentiation markers including Nestin, MAP2, and GFAP in NSCs transfected with GSK3β (S9A) treated with OA (20 μM). NSCs with GSK3β (S9A) significantly suppressed NSC neural differentiation. **p < 0.01, compared with Ad-Ctr + 20 μM OA group. All data are presented as means ± SD from three independent experiments