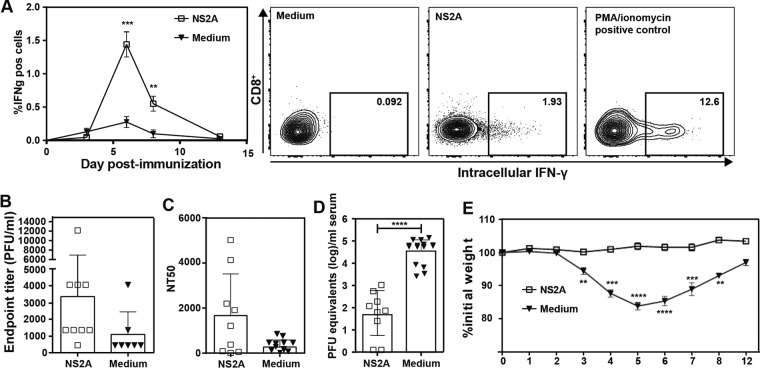
FIG 12.
In vivo efficacy of PIVNS2A. Mice (CD11c-cre × IFNARfl/fl) were immunized with 106 IFU PIVNS2As (i.e., K188-del plus G100VprM plus P179SNS2A plus A373VNS3 produced as described for Fig. 9F) or 10% FCS medium subcutaneously. (A) CD8+ T cell response in the blood was monitored on days 3, 6, 8, and 13. Representative flow cytometry graphs from day 6 are shown on the right. (B) IgG antibody titers after immunization. At 1 month postimmunization, blood was drawn prior to challenge with virulent DENV-2 (strain D2Y98P). The IgG antibody titers against DENV-2 were measured by ELISA. (C) NT50. Neutralizing antibodies against DENV-2 were measured using U937–DC-SIGN cells as target cells. (D) Viremia on day 3 after challenge. After 1 month of immunization, mice were challenged with 107 PFU D2Y98P virus. On day 3 postchallenge, peak viremia was measured by real-time RT-PCR. (E) Weights of mice over the course of 12 days postchallenge. The data are presented as means ± standard deviations. Statistical analysis was performed using Student's t test. **, P < 0.01 (highly significant); ***, P < 0.001 (extremely significant); ****, P < 0.0001. Nonsignificant differences are not indicated. The data are derived from the results of two or three independent experiments with totals of 9 to 15 mice.