Abstract
Soxhlet extraction is a common method of sample preparation. However, there has been no discussion about the efficiency of Soxhlet extraction from different batches and the factors that cause content fluctuation. In this study, Panax ginseng was selected as a model sample. Soxhlet extraction by means of a water bath, which has always been neglected, was identified as a novel key factor in the poor repeatability in different batches of Soxhlet extraction, as it can affect the siphon times and reflux time, which have been positively correlated with the ginsenoside contents. By substituting round bottom flasks in the same column, the relative standard deviation of the most fluctuated compound, ginsenoside Rb1, was decreased from 24.6% to 5.02%. Scanning electron microscopy analysis confirmed that the breakdown of the surface of the ginseng powder in the Soxhlet extraction led to a better dissolution of ginsenosides, indicating that chloroform may promote the extraction of ginsenosides by disrupting the cell structure. Moreover, 70% methanol was regarded as the better solvent for extracting the ginsenosides. Overall, this work offers a practical and effective protocol for improving the accuracy and repeatability of Soxhlet extraction methodology for ginsenosides and other analytes.
Keywords: Ginseng, Ginsenosides, Soxhlet extraction, Repeatability, Siphon times
Graphical abstract
Highlights
-
•
Extraction efficiency evaluation using relationship analysis.
-
•
Positive relationships between siphon/reflux times with ginsenosides.
-
•
Un-parallel soxhlet extraction causes worse repeatability.
1. Introduction
Extraction is an important step in analytical methodologies, as it constitutes the principal source of error and remains one of the most time-consuming one [1], [2], [3]. In practice, it is necessary to select appropriate extractions and solvents based on physic-chemical principles such as polarity and thermal stability, for enriching the desired compounds or removing the impurities [4], [5], [6]. Particularly, Soxhlet extraction, as a dynamic extraction and continuous reflux method, is commonly used for biological, pharmaceutical, food, and environmental analyses [7], [8], [9]. In order to significantly improve the efficiency of sample preparation, batch Soxhlet extraction with separate heat sources has been widely used. However, some unintended processes previously considered negligible, including the heating positions and the devices, have caused significant content fluctuations, yet are rarely reported in batch Soxhlet extraction analysis using the same heat source [3].
Soxhlet extraction is the standard extraction protocol for the quality control of some herbal medicines in China [10] and other countries [11], [12]. In the Chinese Pharmacopoeia 2015 edition for traditional Chinese medicines, the Soxhlet extraction method was used in 33 kinds of medicinal materials for quantitative analysis of plants such as ginseng and red ginseng [10]. Usually, the Soxhlet extraction was adjusted to allow a more complete movement of the grease components [13] and more effective protection of the chromatographic columns than the enzyme hydrolysis [14], ultrahigh pressure extraction [15], microwave-assisted extraction [16], solid extraction [17], and supercritical fluid extraction [18] approaches. However, in this study, we discovered serious ginsenoside content fluctuations in the batch Soxhlet extraction of ginseng and red ginseng using different extraction positions within the same device, which significantly influenced the quantification accuracy.
Considering that batch Soxhlet extraction is a universal issue, Panax ginseng was selected as a model sample to develop a systematic protocol for screening the key factor causing the extraction content fluctuations. Under our developed protocol, the siphon times, reflux time, and corresponding contents in different Soxhlet extraction positions were comprehensively monitored. Association analysis was applied to discover the complex relationship between the negligible factors and the content fluctuations. Scanning electron microscopy (SEM), as a good platform, was also used to discover the mechanism behind the microscopic changes and the ginsenosides release, using different extraction methods [19], [20], [21]. In the United States Pharmacopoeia, methanol solvent is used for the extraction of ginseng, while n-butanol and chloroform are used in the Chinese Pharmacopoeia. Therefore, the microscopic changes in ginseng using different extraction solvents are also discussed in this paper. Association analysis and SEM analysis were used to identify the mechanism behind the content fluctuations in batch Soxhlet extraction for the first time. After removing the interfering factors, we were able to decrease the relative standard deviation of the most fluctuated compound, ginsenoside Rb1, from 24.6% to 5.02%, meeting the provisions of the Chinese Pharmacopoeia [10]. The SEM analysis confirmed that the breakdown of the surface of the ginseng powder in the Soxhlet extraction led to a better dissolution of ginsenoside. This work offers a practical and effective protocol for improving the accuracy and repeatability of the Soxhlet extraction methodology for ginsenosides and other analytes, and proposes for the first time the unique role of chloroform in the extraction of ginsenosides.
2. Materials and methods
2.1. Chemicals and reagents
Reference standards of ginsenosides Rg1 (GRg1, ≥ 91.7%), Re (GRe, ≥ 92.3%), and Rb1 (GRb1, ≥ 93.7%) were purchased from the Chinese National Institutes for Food and Drug Control (Beijing, China). Analytical grade methanol, n-butanol, and chloroform were purchased from Beijing Chemical Works (Beijing, China); HPLC grade methanol and acetonitrile were purchased from Fisher Scientific (Geel, Belgium) and Merck (Darmstadt, Germany), respectively. Deionized water (18.2 MΩ/cm) was prepared by Barnstead GenPure UV/UF water purifier from Thermo Scientific (Langenselbold, Germany).
2.2. Sample preparation
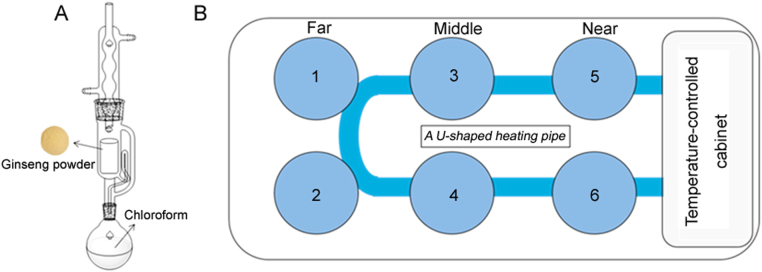
Ginseng and red ginseng samples were purchased from Beijing Tongrentang (Dongzhimen Pharmacy, Beijing, China) in July 2016, and then were dried, crushed over 65 mesh screen (250 ± 9.9 µm), and stored in the dryer. According to the standard extraction of ginseng in the Chinese Pharmacopoeia (2015 Edition), the powders (~ 1 g) loaded into cellulose thimbles were Soxhlet extracted with chloroform (3 h each) using a standard six-hole water bath with 1.5 kW power (Fig. 1). After overnight soaking, the dried ginseng residues along with the filter paper were ultrasonic-extracted (250 W, 50 kHz) with 50 mL water saturated n-butanol for 30 min at room temperature. Then 25 mL subsequent filtrates were evaporated to dryness, dissolved in 5 mL HPLC grade methanol, and mixed well. The solution was filtered through a 0.22-μm PTFE filter from Pall Corporation (Ann Arbor, MI, USA) and stored at 4 °C prior to HPLC analysis.
Fig. 1.
The conventional Soxhlet extractor (A) and standard six-hole water bath (B) for the Soxhlet extraction of ginseng and red ginseng.
2.3. HPLC analysis
All samples were analyzed on an Agilent 1260 HPLC-DAD system (Agilent Technologies, Palo Alto, CA, USA) for qualitative and quantitative analyses of ginsenosides in the ginseng and red ginseng at 30 °C using an Agilent Eclipse XDB-C18 column (4.6 mm × 250 mm, 5.0 µm) with the flow rate of 1 mL/min and the wavelength of 203 nm. The mobile phase consisted of water (A) and acetonitrile (B). The standard gradient elution program was according to the Chinese Pharmacopoeia [10]. This was 19% B at 0–35 min; 19%–29% B at 35–55 min; 29%–29% B at 55–70 min; 29%–40% B at 70–100 min; 40%–100% B at 100–106 min, then 100% B was maintained for 5 min to clean the column. The equilibration time was 5 min.
2.4. Systematic investigations of interference factors
In order to correct for systematic errors in the instruments and natural samples, the methodological parameters of the chemical markers (i.e. GRg1, GRb1, and GRe) including precision, linearity, and stability, were evaluated. Furthermore, the repeatability in the batch Soxhlet extraction was determined by two operators and the stability along with the recovery was also assessed. The intra-day precision was evaluated using the extract of 1 g ginseng powder (n = 6) in one day. The inter-day precision was determined over six successive days by quantification of the same extract. The stock extract was analyzed every 4 h to test the stability of the above three chemical markers at 25 °C. The linearity was assessed at the concentration of 0.0512–0.5120 mg/mL for GRg1, 0.0503–0.5030 mg/mL for GRe, and 0.0537–0.5365 mg/mL for GRb1, respectively. The interference factors in the Soxhlet extraction, including extraction position, siphon time [3], and reflux time [22], were simultaneously determined under the developed sampling method [10] using the same solvent. Finally, association analysis of the ginsenoside contents and these interference factors was comprehensively conducted.
2.5. Scanning electron microscopy (SEM) analysis
Ginseng powders were observed under the SU1510 SEM (Hitachi High-Technologies Corporation, Tokyo Japan) for morphological characterization before and after extraction. Five samples of the untreated residue, the ultrasound extracted residue using 50 mL of 70% methanol (USP Reference Standards) [23], the chloroform refluxed residue, the standard residue using the Chinese Pharmacopoeia [10], and the ultrasound extracted residue using n-butanol were used for the SEM analysis. The study on the role of the solvent in the extraction of ginsenosides from ginseng materials was processed by comparing the effects of different solvents on the morphological changes, which could indicate the ability of the release of intracellular ginsenosides into the extract indirectly. All ultrasound extractions were performed for 30 min at the frequency of 50 kHz and the ultrasonic power of 250 W. After drying the extracted residues at 60 °C in an air oven, all samples were fixed on a specimen holder with a carbon double-sided tape (NISSHIN EM Co., Ltd., Tokyo) and then sputtered with gold in a KYKY SBC-12 sputter-coater (KYKY Technology Co., Ltd., Beijing, China) to be examined with SEM.
3. Results and discussion
3.1. Issues with repeatability in batch Soxhlet extraction
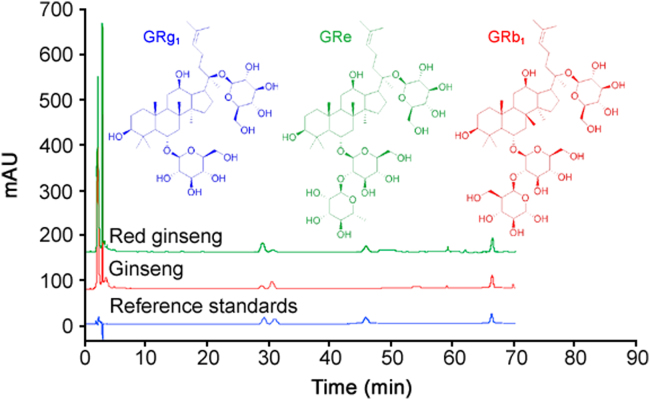
HPLC-UV was used to analyze the ginsenosides in ginseng, in which GRg1 (28.9 min), GRe (30.8 min), and GRb1 (46.2 min) displayed good peak shapes and separations (Fig. 2). Under the set concentration ranges, the squared multiple correlation coefficients (R2) of all determined ginsenosides were above 0.9991, and the intra-day and inter-day precisions were all below 4.43% (Table 1), indicating the accuracy of the instrument and the adopted separation method was sufficient. In addition, all extracted samples were stable for 36 consecutive hours and monitored every 4 h at 25 °C.
Fig. 2.
The HPLC spectra of ginseng, red ginseng, and mixed reference standards.
Table 1.
The linearity, precision, and stability of the official HPLC-UV for ginseng.
| Component | Curvilinear equation | R2 | Linearity range (mg/mL) | Precision (%, RSD) |
Stability (%, RSD) | |
|---|---|---|---|---|---|---|
| Inter-day | Intra-day | |||||
| GRg1 | Y = 3115.4X − 10.8830 | 0.9998 | 0.0512–0.5120 | 2.20 | 1.66 | 3.03 |
| GRe | Y = 2750.6X − 14.5050 | 0.9997 | 0.0503–0.5030 | 0.28 | 2.06 | 1.26 |
| GRb1 | Y = 2287.6X + 3.8394 | 0.9991 | 0.0537–0.5365 | 0.60 | 4.43 | 2.26 |
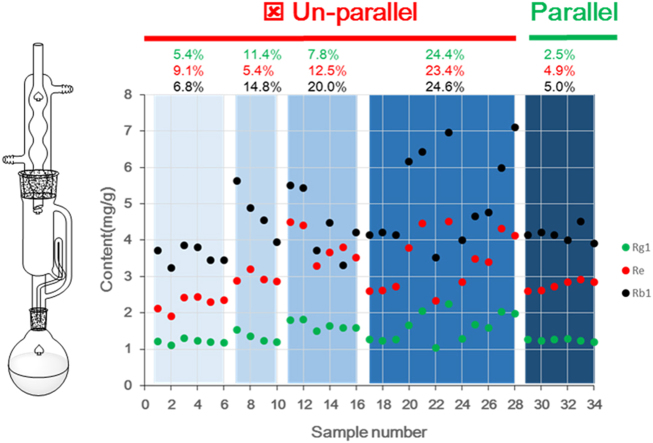
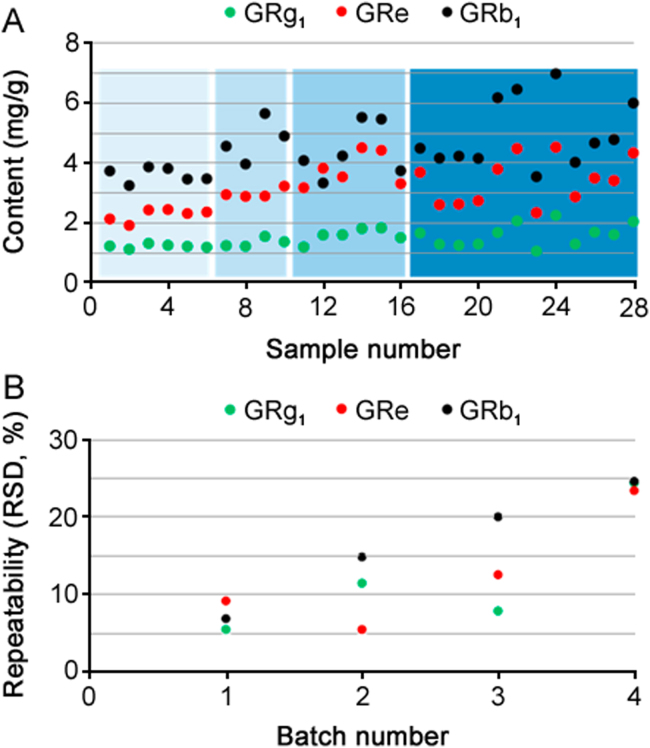
However, poor repeatability always appeared in the batch Soxhlet extraction with different positions. Initially, these significant quantification fluctuations were speculated to be caused by artificial errors, so two operators, the No. 1–10 samples for the one and the No.11–16 samples for the other, conducted repeatability studies on different days (Fig. 3A). However, the repeatability was still unsatisfactory. Traditionally, the use of more samples (9 batches, No. 17–28) could effectively eliminate the errors. Surprisingly, the relative standard deviation of the most variable, ginsenoside Rb1, even reached 24.6% (Fig. 3B), indicating that more samples led to poorer quantification. Notably, the similar content change trends for GRb1 and GRg1 indicated that the instability of the ginsenosides was not the responsible factor. Thus, considering the good credibility of ultrasonic extraction followed by HPLC for ginsenosides [24], we began to speculate that some other factors in the Soxhlet extraction might be responsible for the poor repeatability.
Fig. 3.
The determined contents (A) and their corresponding repeatability (B) of ginsenosides. Especially, the No. 1 – 10 and No. 17 – 28 results were examined by the same operator on different days, and the No. 11 – 16 results were examined by another operator.
3.2. Systematic association analyses of the Soxhlet extraction factors with the content fluctuations
While processing the repeatability test, we found the reflux time and droplet speed were different for the extraction holes in the far (Positions 1 and 2), middle (Positions 3 and 4), and near (Positions 5 and 6) positions in the U-type temperature regulator (Fig. 1B, and Table 2). Moreover, the fastest droplet speed extracted more ginsenosides. In order to elucidate the complex relationship of the contents and the reflux time, the efficiency of all selected round flasks was first evaluated using the same volume of chloroform (~ 200 mL) under the conventional Soxhlet extractor (Fig. 1A) and consecutively tested 3 h in the same hole. When there were no significant differences for the mean reflux time per 100 drops of chloroform [24] and siphon times [3], the round flask would be selected to conduct further studies.
Table 2.
Extraction efficiency of ginsenosides at different positions of the standard six-hole water bath.
| Position | Reflux time (s/100 drops) | Siphon times (in 3 h) | Red ginseng |
Ginseng |
||||
|---|---|---|---|---|---|---|---|---|
| GRg1 (%) | GRe (%) | GRb1 (%) | GRg1 (%) | GRe (%) | GRb1 (%) | |||
| 1 | 44 | 6.5 | 0.29 | 0.08 | 0.33 | 0.12 | 0.23 | 0.34 |
| 2 | 44 | 6.5 | 0.29 | 0.08 | 0.34 | 0.12 | 0.23 | 0.35 |
| 3 | 28 | 8.0 | 0.31 | 0.09 | 0.40 | 0.13 | 0.24 | 0.39 |
| 4 | 29 | 8.0 | 0.29 | 0.08 | 0.38 | 0.12 | 0.24 | 0.38 |
| 5 | 54 | 3.0 | 0.26 | 0.08 | 0.28 | 0.12 | 0.21 | 0.37 |
| 6 | 54 | 3.0 | 0.26 | 0.07 | 0.29 | 0.11 | 0.19 | 0.32 |
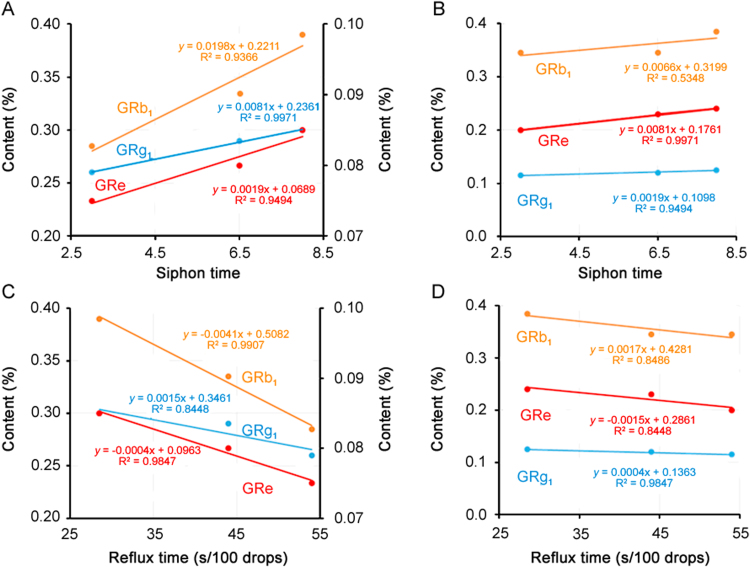
Unfortunately, using the same parameters, significant differences still appeared when the selected round flasks were used in different positions (Table 2). The samples from the two middle holes had the smallest mean reflux time to reach 100 drops, resulting in the largest siphon times, with 8.0 in 3 h. Moreover, the reflux time of the far holes were larger than those of the near ones. Meaningfully, the middle holes of the Soxhlet extractor showed the highest efficiency for sampling. The samples from the same column of holes had similar extraction efficiency (RSD < 5%). Interestingly, for the first time, using association analysis, we discovered a strong relationship between the siphon times and ginsenosides contents in ginseng and red ginseng (Figs. 4A and B). Their R2 values of the contents with the siphon times were all above 0.5348 in red ginseng and 0.8448 in ginseng, and even reached 0.9847 for GRe or GRg1 in different types of samples. Moreover, the results between the reflux time per 100 drops with the contents confirmed the above good relationships (their R2 all above 0.8448) (Figs. 4C and D), indicating the different positions of the Soxhlet extractor containing the U-type temperature regulator were the major factor causing poor repeatability. Meaningfully, the different extraction efficiencies of the different water-bath positions would significantly influence the accuracy of the quantification for ginsenosides, which was considered a negligible factor in previous studies. At the same time, it also suggested that the different positions of one water bath could have an effect on the extraction efficiency of ginsenosides. Therefore, more attention should be paid to the design of the temperature regulator for the water bath [3] in future batch Soxhlet extractions.
Fig. 4.
The positive relationship between the siphon times (A and B) and reflux time (C and D) for ginseng (left) and red ginseng (right) samples.
3.3. Accurate quantification of ginsenosides using the same column positions for the Soxhlet extraction
According to the above results, we used parallelized round bottom flasks to extract samples in the same column positions, the middle two holes (Positions 3 and 4), to re-determine the recovery and repeatability (Table 3). Fortunately, the recovery ranged from 95.29% to 106.88% and the repeatability was below 5.02%, indicating that this new method could meet the requirements of the Chinese Pharmacopoeia [10]. Moreover, this unintended extraction process solidly for the first time confirmed that the water-bath position for the Soxhlet extraction was critical to accurately quantify the ginseng ginsenosides. In tests of real samples, their concentrations of GRg1, GRe, and GRb1 in ginseng were 0.12%, 0.22%, and 0.36%, and in red ginseng were 0.30%, 0.09%, and 0.39%, respectively. Notably, in the comparison of the above complex optimized official sample preparation, simple ultrasonic extraction [24], [25], with a short extraction time, low solvent consumption, and good repeatability, is another choice for the accurate quantification of ginsenosides in future study.
Table 3.
The recovery and repeatability of parallel soxhlet extraction for ginsenosides.
| Component | Recovery (%, n = 9) |
Repeatability (%, RSD, n = 6) | ||
|---|---|---|---|---|
| Low (80%) | Med. (100%) | High (120%) | ||
| GRg1 | 104.44 | 97.25 | 103.51 | 2.47 |
| 97.43 | 102.05 | 98.83 | ||
| 106.88 | 101.95 | 99.39 | ||
| GRe | 99.06 | 98.01 | 95.71 | 4.90 |
| 104.15 | 101.27 | 98.24 | ||
| 98.88 | 102.55 | 95.29 | ||
| GRb1 | 96.67 | 106.21 | 104.09 | 5.02 |
| 100.83 | 99.77 | 100.77 | ||
| 95.84 | 97.50 | 103.31 | ||
3.4. Scanning electron microscopy for discovering the Soxhlet extraction mechanism for improving ginsenosides release from materials
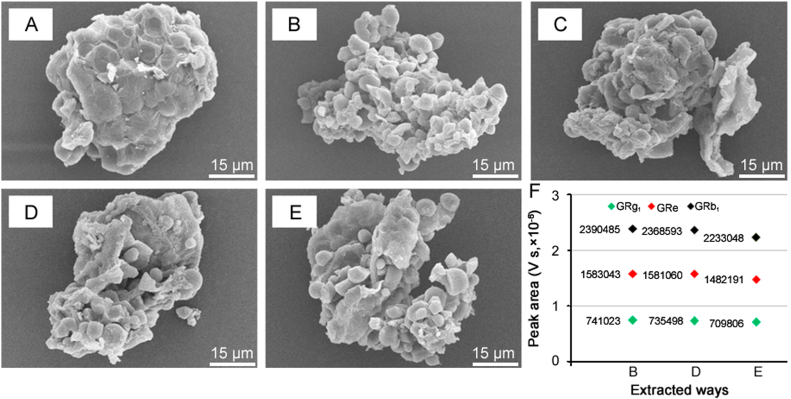
In order to elucidate how the conventional Soxhlet extractor assists the extraction of ginsenosides from raw ginseng, SEM was used to examine the surface changes that could account for the sampling-triggered content release [21], [26]. Compared with the untreated sample, there were serious structure changes on the surfaces of the samples treated using 70% methanol, chloroform, chloroform along with n-butanol, and n-butanol only (Figs. 5A-E), which were selected according to the ginseng extraction method in the U.S. Standard and Chinese Pharmacopoeia [10]. After Soxhlet extraction using chloroform only, slight cellular damage with some small particles was observed (Figs. 5A and D). In addition, the Soxhlet extraction effectively assisted sonication by disrupting the surface structure of the ginseng powder and rapidly releasing intracellular ginsenosides (Figs. 5B and D-F), which is the main by which Soxhlet extraction improves the extraction of ginsenosides. Therefore, the degree of damage to the surface wall of the ginseng powder was increased after being Soxhlet extracted by chloroform. In other words, it allowed more ginsenosides from the raw materials to be extracted by the following n-butanol. Notably, the significant differences in the penetrability and enrichment ability of the solvents in regards to the cell walls caused serious damage to the treated samples and varied concentrations, respectively. The chloroform reflux process will inevitably lead to the loss of effective components [27]. However, in our study, the ginsenoside contents at larger siphon times were higher than those at smaller siphon times, indicating that the loss of ginsenosides was less than that using ultrasonic-extraction with water saturated n-butanol. Therefore, for ginseng, chloroform promoted the extraction of ginsenosides by destroying the cell structure and not only the usual degreasing. Notably, the yields of ginsenosides ultrasoundly extracted with 70% methanol were higher than those of the chloroform reflux with n-butanol ultrasound (Fig. 5F), indicating the 70% methanol was the better solvent for extracting the ginsenosides.
Fig. 5.
Scanning electron microscopic (SEM) analyses and ginsenoside content comparisons using different sampling methods. The SEM images of the residues for untreated sample (A) and treated samples using ultrasound extraction with 70% methanol for 30 min (B), chloroform reflux for 3 h (C), chloroform reflux for 3 h along with n-butanol ultrasound extraction for 30 min (D), and n-butanol ultrasound extraction for 30 min (E) were recorded. The mean peak areas of ginsenosides (n = 3) of the three ways of extraction were compared (F). In particular, the peak area of sample B was zoomed in five times due to the fact that the solvent volume was 50 mL for 1 g ginseng powder.
4. Conclusion
This study demonstrates a standard protocol that systematically investigates some important factors of batch Soxhlet extraction resulting in content fluctuations. Interestingly, we were the first who discovered the different water-bath positions of the conventional Soxhlet extractor, considering a negligible factor in previous studies, could significantly cause the poor repeatability of ginsenosides. In particular, the unstable ginsenoside Rb1 was above 24.6% using batch Soxhlet extraction. After processing the developed protocol, different Soxhlet extraction positions of the same device showed significant differences in efficiency. In addition, using correlation analysis for the first time, the reflux time and siphon times were found to be in good linear agreement with their corresponding ginsenoside contents. Therefore, the water-bath position of the Soxhlet extraction was a key factor assisting the extraction of ginsenosides from raw ginseng material. In addition, SEM confirmed that Soxhlet extraction improves ultrasound extraction by disrupting the surface of the ginseng powder to effectively release the cellular ginsenosides, not just the previously considered defatting. In real sample analyses, the recovery and repeatability in the same column positions at the middle holes could meet the requirements of the Chinese Pharmacopoeia, indicating that the efficiency of different Soxhlet extraction positions should be systematically investigated in further studies. Furthermore, the content results of the official sample preparations showed that ultrasonic extraction with 70% methanol was feasible and credible for the accurate quantification of the ginsenosides in ginseng.
Acknowledgments
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The financial support of the key project at the central government level: The Ability Establishment of Sustainable Use for Valuable Chinese Medicine Resources (No. 2060302), the Independent Research Grant of National Resource Center for Chinese Materia Medica, China Academy of Chinese Medical Sciences (No. ZZXT201608), and the National Natural Science Foundation of China (No. 81603293), was appreciated.
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Contributor Information
Lu-Qi Huang, Email: huangluqi01@126.com.
Chang-Jiang-Sheng Lai, Email: laichangjiang44@126.com.
References
- 1.Chen R., Meng F., Zhang S. Effects of ultrahigh pressure extraction conditions on yields and antioxidant activity of ginsenoside form ginseng. Sep. Purif. Technol. 2009;66:340–346. [Google Scholar]
- 2.Huie C.W. A review of modern sample-preparation techniques for the extraction and analysis of medicinal plants. Anal. Bioanal. Chem. 2002;373:23–30. doi: 10.1007/s00216-002-1265-3. [DOI] [PubMed] [Google Scholar]
- 3.Luque de Castro M.D., Priego-Capote F. Soxhlet extraction: past and present panacea. J. Chromatogr. A. 2010;1217:2383–2389. doi: 10.1016/j.chroma.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 4.Yang X., Fan S.C. Progress in traditional Chinese medicine extraction method. Asia-Pac. Trad. Med. 2012;8:194–196. [Google Scholar]
- 5.Mustafa A., Turner C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta. 2011;703:8–18. doi: 10.1016/j.aca.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Oniszczuk A., Podgórski R. Influence of different extraction methods on the quantification of selected flavonoids and phenolic acids from Tilia cordata inflorescence. Ind. Crop Prod. 2015;76:509–514. [Google Scholar]
- 7.Ryu J., Lee H.W., Yoon J. Effect of hydrothermal processing on ginseng extract. J. Gins. Res. 2017;41:572–577. doi: 10.1016/j.jgr.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Y., Zheng J., Gan R.Y. Optimization of ultrasound-assisted extraction of antioxidants from the Mung Bean Coat. Molecules. 2017;22:638. doi: 10.3390/molecules22040638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cloutier P.L., Fortin F., Groleau P.E. QuEChERS extraction for multi-residue analysis of PCBs, PAHs, PBDEs and PCDD/Fs in biological samples. Talanta. 2017;165:332–338. doi: 10.1016/j.talanta.2016.12.080. [DOI] [PubMed] [Google Scholar]
- 10.National Commission of Chinese Pharmacopoeia, Pharmacopoeia of the People’s Republic of China, 2015 ed., Beijing, China, 2015.
- 11.European Directorate for the Quality of the Medicines (EDQM), European Pharmacopoeia 8.0, Strassbourg, France, 2014.
- 12.Masala S., Rannug U., Westerholm R. Pressurized liquid extraction as an alternative to the Soxhlet extraction procedure stated in the US EPA method TO-13A for the recovery of polycyclic aromatic hydrocarbons adsorbed on polyurethane foam plugs. Anal. Methods. 2014;6:8420–8425. [Google Scholar]
- 13.Zhou L.N., Hao W.L., Wu S.L. Effects of different accelerated solvent extraction conditions on lipids extraction from Chlorella sorokiniana. Guangdong Agr. Sci. 2016;43:126–132. [Google Scholar]
- 14.Lai C.J.S., Tan T., Zeng S.L. An enzymatic protocol for absolute quantification of analogues: Application to specific protopanoxadiol-type ginsenosides. Green. Chem. 2015;17:2580–2586. [Google Scholar]
- 15.Zhang S., Chen R., Wu H. Ginsenoside extraction from Panax quinquefolium L. (American ginseng) root by using ultrahigh pressure. J. Pharm. Biomed. Anal. 2006;41:57–63. doi: 10.1016/j.jpba.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 16.Shu Y.Y., Ko M.Y., Chang Y.S. Microwave-assisted extraction of ginsenosides from ginseng root. Microchem. J. 2003;74:131–139. [Google Scholar]
- 17.Xu X., Liang S., Meng X. A molecularly imprinted polymer for the selective solid-phase extraction of dimethomorph from ginseng samples. J. Chromatogr. B. 2015;988:182–186. doi: 10.1016/j.jchromb.2015.02.033. [DOI] [PubMed] [Google Scholar]
- 18.Oba C., Ota M., Nomura K. Extraction of nobiletin from Citrus Unshiu peels by supercritical fluid and its CRE-mediated transcriptional activity. Phytomedicine. 2017;27:33–38. doi: 10.1016/j.phymed.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Faltermaier A., Zarnkow M., Becker T. Common wheat (Triticum aestivum L.): evaluating microstructural changes during the malting process by using confocal laser scanning microscopy and scanning electron microscopy. Eur. Food Res. Technol. 2015;241:239–252. [Google Scholar]
- 20.Dahmoune F., Nayak B., Moussi K. Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. leaves. Food Chem. 2015;166:585–595. doi: 10.1016/j.foodchem.2014.06.066. [DOI] [PubMed] [Google Scholar]
- 21.Liu J., Liu Y., Zhang Z.H. Correlation of cultivation time of Panax ginseng with metabolic profiles of nine ginsenosides and mRNA expression of genes encoding major biosynthetic enzymes. Acta Physiol. Plant. 2016;38:51. [Google Scholar]
- 22.Lv W.F., Ding M.Y., Zheng R. Isolation and quantitation of amygdalin in Apricot-kernel and Prunus tomentosa Thunb. by HPLC with solid-phase extraction. J. Chromatogr. Sci. 2005;43:383–387. doi: 10.1093/chromsci/43.7.383. [DOI] [PubMed] [Google Scholar]
- 23.The United States Pharmacopeial Convention, herbal medicines compendium, Panax ginseng Steamed Root and Rhizome of Version 0. 2, United States, 2016, 〈https://hmc.usp.org/monographs/panax-ginseng-steamed-root-and-rhizome-0-2〉.
- 24.Zhang B., Yang R., Liu C.Z. Microwave-assisted extraction of chlorogenic acid from flower buds of Lonicera japonica Thunb. Sep. Purif. Technol. 2008;62:480–483. [Google Scholar]
- 25.Li S.P., Qiao C.F., Chen Y.W. A novel strategy with standardized reference extract qualification and single compound quantitative evaluation for quality control of Panax notoginseng used as a functional food. J. Chromatogr. A. 2013;1313:302–307. doi: 10.1016/j.chroma.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 26.Kala H.K., Mehta R., Sen K.K. Strategizing method optimization of microwave-assisted extraction of plant phenolics by developing standard working principles for universal robust optimization. Anal. Methods. 2017;9:2089–2103. [Google Scholar]
- 27.Dong W., Qian Y., Zhu X. Determination of ginsenosides at the presence of phospholipids. J. China Pharm. Univ. 1995;26:282–285. [Google Scholar]