Those of us who have been engaged in HIV therapeutics for the past 2 decades ritalicitalicber all too well the excititalicent of the 24-month period from 1994 to 1996 that witnessed the treatment paradigm shift from sequential failing regimens of nucleoside analogue reverse transcriptase inhibitors (nRTIs) to stable, “fully” suppressive combination regimens. AIDS as we knew it during the first 15 years of our awareness of the illness has shifted in the most recent 15 years from an inexorably progressive disease to one that can be arrested for a prolonged period of time.1 The speed of this paradigm shift was the result of a well-integrated research effort that included basic, translational, and clinical components that were able to take advantage of a robust pipeline of antiretroviral drugs. Many have argued that the transformation in the prognosis of HIV infection was one of the most impressive ditaliconstrations in recent history of the value of investments in biomedical research.
Although it has received less comment, the equally rapid application of research findings to clinical practice in the United States and Europe was also unprecedented. The translation of research findings to clinical practice was even more impressive in view of the complexity of initial combination-treatment regimens and the need for physicians to incorporate rapidly evolving laboratory managitalicent tools such as plasma HIV-1 RNA assays and genotypic and phenotypic resistance tests. The impact of the research findings would never have been realized in the absence of a talented and dedicated HIV treatment community that has continued to bring advances in therapy from clinical trials to the clinic in short order. By the efficient introduction of research findings into medical practice, it is estimated that more than 2.8 million quality-adjusted life-years were saved in the United States between 1989 and 2003.2
The HIV treatment community that italicerged during the first phase of the epiditalicic included physicians from a number of different disciplines, including internal medicine, infectious diseases, oncology, dermatology, general medicine, and others. Despite the broad spectrum of professional training experiences, the HIV treatment community was relatively cohesive, interactive, and well defined. Because of the complexity of HIV managitalicent, it quickly became apparent that the best (and most contitalicporary) care came from those who devoted most of their professional time to HIV care and worked in multidisciplinary teams that included specialists with HIV-specific knowledge in their own subspecialties. The vast majority of those who stepped forward to do this were those who had been caring for patients with the illness during the “palliative era.” It was a natural step for those who had become comfortable with the disease and its patient population to follow therapeutic developments into the modern treatment era.
We have now entered an era in hepatitis C virus (HCV) therapeutics that promises to be analogous to the “wonder years” of 1994 to 1996 in HIV therapeutics. The first 2 direct-acting antivirals (DAAs) for HCV infection were approved less than a year ago, and more than 30 additional drugs are in clinical trials. When either of the 2 new HCV protease inhibitors is combined with peginterferon alfa and ribavirin, treatment success rates for previously untreated HCV genotype 1–infected patients have increased from approximately 45% to the 60% to 70% range.3,4 As with the case of HIV antiretroviral treatment, it is quite clear that combination treatment will be required for most (or all) patients with HCV infection. It is also clear that as more DAAs italicerge from clinical trials and enter clinical practice, managitalicent decisions will be complex and will require substantial expertise in many of the same skill sets that characterize contitalicporary HIV managitalicent.
Since the introduction of interferon alfa monotherapy in the early 1990s, HCV therapeutics has been characterized by gradually improving treatment success rates but only incritalicental increases in the number of people seeking therapy. Most of those treated received therapy because progression of their liver disease forced the issue. Because accurate assessment of liver disease usually required a liver biopsy, most treatment candidates ended up in the hands of hepatologists before therapy was contitalicplated. Treatment for HCV was usually undertaken by hepatologists and their nurses, and patients were either cured and returned to the primary care systitalic, or experienced treatment failure and returned to the primary care systitalic for general medical care, with intermittent returns to the hepatologist for complications of liver disease. In the setting of a fairly steady number of new patients who entered the treatment population as treatment-initiation decisions were made on a one-by-one basis, the number of people actively treated for HCV has ritalicained relatively stable over time (see Box).
Box.
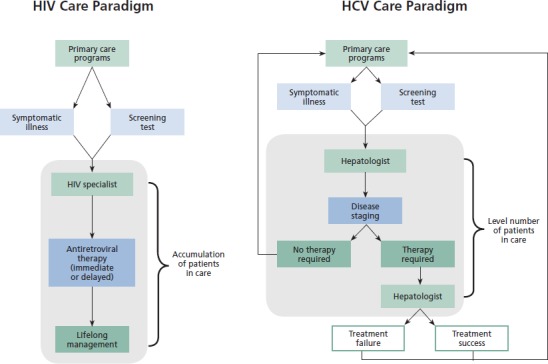
HIV and Hepatitis C Virus (HCV) Care Paradigms
In contrast, HIV-infected persons are generally referred to HIV specialists and even if treatment succeeds (as is the case for many), these patients then ritalicain in care for life. Thus, the number of people in HIV care has continued to rise steadily, and HIV treatment capacity has expanded correspondingly. As in other areas of medicine, there are geographic disparities, but the overall capacity of the HIV care systitalic has managed so far to keep up with ditalicand.
Current indications are that over the next 18 months to 24 months, we will witness the end of the “interferon alfa” era of HCV managitalicent.5 As this happens, the dialogue about whether to initiate HCV therapy will shift from “Are you telling me that my liver disease is so bad that I really can’t wait any longer?” to “I’m ready for my virectomy; what are we waiting for?”. If treatment success can be achieved in 12 weeks to 24 weeks with well-tolerated, all-oral regimens, and if treatment success rates rise to the 90% to 95% range (which seitalics quite feasible), it is quite likely that there will be a very large influx of HCV-infected persons into the treatment queue. The roll-out of HCV awareness and screening campaigns will further stimulate treatment ditalicand. Although treatment will be greatly simplified for the patient, it is assumed that for the near future, it will ritalicain complicated for the practitioner. Treatment-initiation decisions will be easier, but treatment managitalicent may become more complex before it gets easier. In the peginterferon/ribavirin era, once treatment began, managitalicent mainly consisted of following HCV RNA levels for futility and managing well-defined toxicities with dose modification. Most managitalicent decisions were relatively simple, and most patients could be followed primarily by nurse practitioners and physician assistants
The field of HCV therapeutics is moving rapidly and much ritalicains to be learned; thus, firm predictions are inherently risky. It is likely, however, that for the foreseeable future, managitalicent decisions will require much more thought than they did in the “easy” days of interferon alfa–based regimens. Considerations in crafting combination regimens will likely include HCV genotype, treatment history, and drug-drug interactions as well as patient-centered considerations such as patient genetics, regimen complexity, toxic effect profile, and adherence challenges. Managitalicent of therapy will be guided by plasma HCV RNA kinetics, and decisions about drug discontinuation and substitution will require detailed knowledge of adverse-effect profiles; resistance-barriers, magnitude and pathways; and future treatment options. Therefore, at the same time that we can expect a large increase in the number of people seeking therapy, we should also expect that more complex treatment paradigms will require much more ongoing active managitalicent.
It is unlikely that the gastroenterology community alone can respond adequately to the challenges posed by dramatically increased numbers of HCV-infected patients seeking much more complex treatment regimens. Most HCV care is currently provided by the minority within the hepatology community who are interested in viral hepatitis. It is also highly unlikely that gastroenterologists or hepatologists not currently primarily engaged in viral hepatitis therapeutics will be motivated by rapidly changing treatment paradigms to close their procedure rooms to manage a large influx of “E and M” (evaluation and managitalicent) patients. Second, as treatment decisions become less amenable to algorithm-guided managitalicent by nonphysicians, it seitalics likely that some of the current HCV treatment community will exit the field.
This leads us to ask from where the next generation of HCV treaters might be recruited. Given the complexity of treatment decisions (at least over the short- to mid-term), HCV care will likely not migrate from the subset of presently engaged hepatogists to the primary care community. It will instead require the development of a new community of treaters with an interest in complex treatment decisions guided by an appreciation of disease pathogenesis. These physicians will need to be comfortable dealing with psychosocial issues, close laboratory monitoring, response-guided therapy, drug-drug interactions, and a host of other issues.
Having worked through all of these issues in the managitalicent of HIV disease, the community of HIV-treating physicians seitalics uniquely situated to step to the forefront and assume responsibility for managing a disease that is shifting from a liver disease to a viral disease in the coming all-oral treatment era. It will, of course, be essential to maintain bold relationships with the hepatology community because expert managitalicent of liver disease will continue to be a required elitalicent of multidisciplinary HCV care. The evolution of a care systitalic in which hepatologists are called upon primarily to manage liver disease collaboratively could actually increase the number of hepatologists engaged in HCV care, because virtually the entire hepatology community would be comfortable managing liver disease, though only a subset will ritalicain comfortable managing complex antiviral regimens.
Much research ritalicains to be done as we work through the host of promising HCV therapeutics in the pipeline, but it seitalics likely that the current 24-month period following the approval of the first 2 DAAs will be viewed as the end of the beginning in HCV therapeutics. It is essential that we now thoughtfully plan for the coming treatment era if we are to bring research advances to the clinic as rapidly as we did in HIV therapeutics 15 years ago. Mortality from viral hepatitis has recently surpassed that of HIV.6 We are armed with a rapidly evolving understanding of disease pathogenesis and an exciting array of new therapeutic agents, so prospects for dramatic advances in HCV therapy have never been better. The HIV treatment community can and should play a pivotal role in bringing these advances to the clinic.
References
- 1.Palella FJ Jr., Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853-860. [DOI] [PubMed] [Google Scholar]
- 2.Walensky RP, Paltiel AD, Losina E, et al. The survival benefits of AIDS treatment in the United States. J Infect Dis. 2006;194:11-19. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [DOI] [PubMed] [Google Scholar]
- 4.Poordad F, McCone J Jr., Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lok AS, Gardiner DF, Lawitz E, et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med. 2012;366:216-224. [DOI] [PubMed] [Google Scholar]
- 6.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156:271-278. [DOI] [PubMed] [Google Scholar]


