Fig. 4. Orally administered SphK2-specific inhibitor decreases the fatality observed in IAV-infected mice.
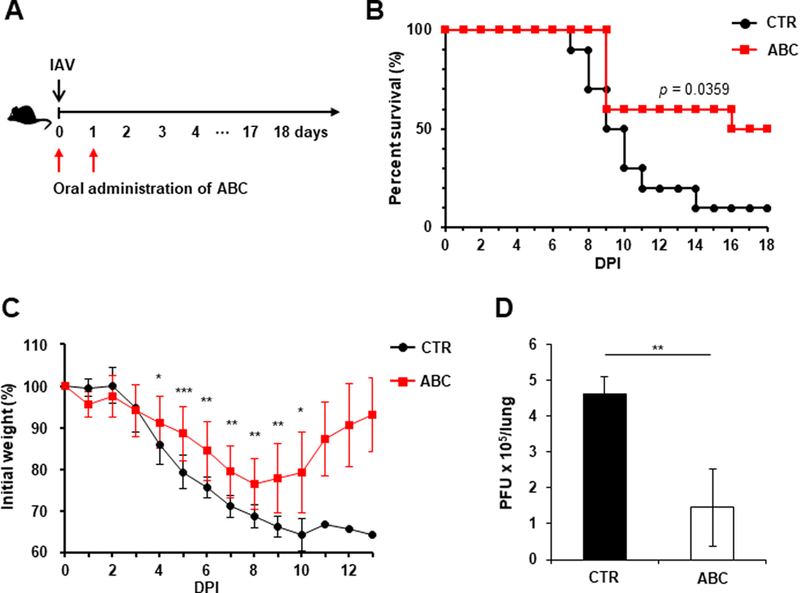
(A-B) C57BL/6 mice were infected with influenza A/PR/8/34 virus (IAV) intranasally (i.n.) at 1×103 PFU. Then, mice were orally administered solvent (PBS) control (CTR) (n=10) or ABC294640 (ABC; 75 mg/kg) daily for 2 days (day 0 and day 1; n=10). All groups were monitored daily for survival. The p value is shown (Log-rank test). (C) The body weights of the mice from (B) were measured daily from day 0 to day 13. The data represent means ± SD (*, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001). (D) C57BL/6 mice were infected with IAV intranasally at 1×103 PFU. The mice were then orally administered PBS control (CTR, n=4) or ABC (n=4) for 2 consecutive days starting from day 0. Lungs were collected at 3 dpi and viral titers were determined by plaque assay. The data represent means ± SD. **, p ≤ 0.01.
