Figure 2.
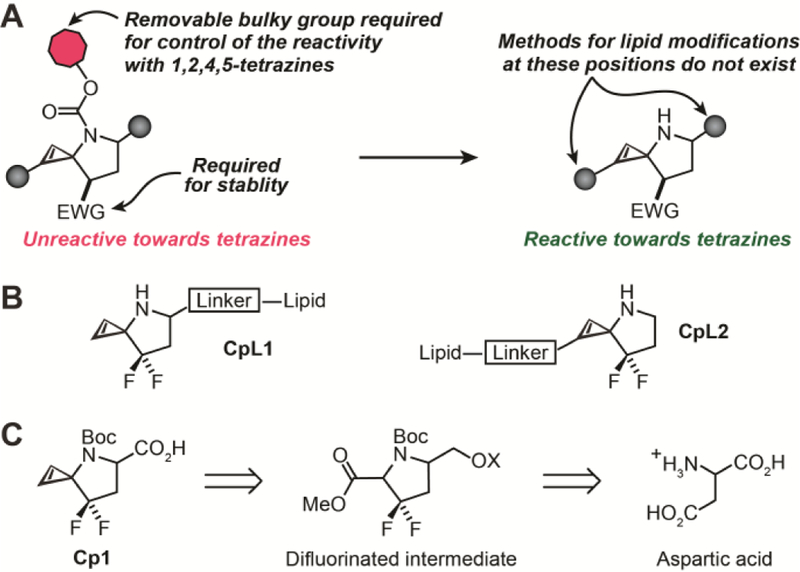
(A) Photocaged 3-N cyclopropenes allow light-mediated control of cyclopropene-tetrazine bioorthogonal ligation. Such 3-N cyclopropene formation require an EWG to prevent rearrangement to an alkyne or allene. Installing a linker on the EWG-stabilized 3-N spirocyclopropenes will enable an alternative to currently available C3 modification for attaching lipids (or other biomolecules) to cyclopropenes. (B) Lipids can be attached to 3-N cyclopropenes either at C1/C2 or on the pyrrolidine ring (C) Retrosynthetic analysis for Cp1 identified aspartic acid as an inexpensive starting point with the ‘difluorinated intermediate’ as a key scaffold.
