Abstract
Background
Studies have shown that intermittent hypoxia mimics obstructive sleep apnea in causing pulmonary inflammation, but the mechanism is not yet clear.TLR-4 is a recognized proinflammatory factor, so the purpose of this study was to assess the function of TLR-4 in pulmonary inflammation induced by chronic intermittent hypoxia simulating obstructive sleep apnea.
Material/Methods
Healthy male Wistar rats were divided into 3 groups (8 in each group): the normoxia control group (CG), the intermittent hypoxia group (IH), and the TLR4 antagonist TAK242 treatment group (3 mg/kg, daily), with exposure durations of 12 weeks and 16 weeks (HI). The morphological changes of lung tissue were determined with hematoxylin-eosin (HE) staining. The expressions of the TLR-4 pathway in lung tissue were tested by Western blotting and RT-PCR. The levels of IL-6 and TNF-α in serum and lung tissue were detected by enzyme-linked immunosorbent assay (ELISA). The levels of SOD and MDA in lung tissue were detected by use of SOD and MDA kits, respectively.
Results
After TAK242 treatment, damage to lung tissue was increased, and the expressions of TLR-4, MYD88, P65, IL-6, TNF-α, MDA, and SOD were decreased. Intermittent hypoxic exposure caused alveolar expansion, thickening of alveolar septum, and fusion of adjacent alveoli into larger cysts under intermittent hypoxia in a time-dependent manner. Compared with the CG and HI groups, the mean lining interval (MLI) become more thickened and the alveolar destruction index (DI) increased significantly in the IH group.
Conclusions
Chronic intermittent hypoxia causes pulmonary inflammatory response and the inflammatory pathway involved in TLR4 receptor may be one of the mechanisms that trigger lung inflammation.
MeSH Keywords: NF-kappa B; Pulmonary Disease, Chronic Obstructive; Sleep Apnea Syndromes; Toll-Like Receptor 4
Background
Obstructive sleep apnea syndrome (OSAS) is a common chronic respiratory disease. Chronic intermittent hypoxia (CIH) is the major pathophysiological feature of OSAS. It has been reported that the transient and rapid progression of hypoxia-reoxygenation in CIH, a typical pathophysiological process of OSAS, is somewhat similar to ischemia-reperfusion injury [1]. Some studies have found that during this hypoxic/reoxygenation pathological process of ischemia-reperfusion, many reactive oxygen species (ROS) are produced, which leads to oxidative stress in the body [2,3]. At the same time, reactive oxygen species (ROS) and peroxides as second messengers can initiate and regulate the oxidation, reducing activity of sensitive signaling pathways and transcription factors such as hypoxia-inducible factor (HIF-1), nuclear factor κB (NF-κB), and activin-1 (AP-1) [4]. Among these, NF-κB is an important inflammatory reaction initiation factor [5] that can be activated in the absence of oxygen and increases with the gradual extension of hypoxia time. OSAS patients with increased expression of NF-κB, the downstream inflammatory mediators and cytokines such as tumor necrosis factor-α, IL-6, and IL-8, etc also increased, and then lead to inflammatory reactions and inflammatory waterfall effect.
OSAS-induced inflammatory processes and related diseases may be caused by NF-κB activation [6]. Studies have shown [7] that cardiac dysfunction caused by CIH is associated with increased cardiac inflammation, apoptosis and increased fibrosis due to increased molecular expression of HIF-1α and NF-κB signaling. At the same time, some researchers have proposed [8] that NF-kB can induce the expression of HIF-1αmRNA while the HIF-1a promoter contains an active NF-κB binding site at -197/188 upstream of the transcriptional start site with intermittent low oxygen activates both HIF-1 and NF-kB transcription factors. Therefore, activation of NF-kB may play an important role in various diseases caused by CIH.
Toll-like receptor 4 is an innate immune receptor present on the surface of immune cells and plays an important role in activating signal transduction. It is also a bridge between innate immunity and adaptive immunity [9]. TLR-4 plays a key role in innate and adaptive immunity by generating a large number of cytokines and proinflammatory cytokines via the MyD88-dependent (MyD88) -dependent and MyD88-independent pathways [10]. The classic signal transduction pathway is MyD88-dependent, which initiates downstream inflammatory responses primarily through mediating the production of proinflammatory cytokines and transcription of NF-κB [11]. More and more studies have found that TLR-4/NF-κB signaling is important in the production and progression of inflammation [12,13]. One study showed a significant increase in TLR-4 expression on monocytes in OSAS patients [14]. TLR4 deletion in mice prevented CIH-induced aortic inflammatory changes [15]. Toll-like receptor-4 is involved in the development of ischemia-reperfusion injury in the heart, liver, kidney and brain models. Similarly, TLR-4 plays a pivotal role in the development of lung ischemia-reperfusion injury [16]. The typical features of OSAS CIH approximate the process of ischemia-reperfusion injury. And recent studies have shown that intermittent hypoxia mimics obstructive sleep apnea causing pulmonary inflammation [17]. To the best of our knowledge, there are no publications showing the role of TLR4 in intermittent hypoxia-induced lung inflammatory responses. Therefore, we hypothesize that the TLR-4/NF-κB pathway may play a key role in lung inflammation induced by OSAS and that TLR-4 antagonist may reduce lung injury and inflammatory response induced by CIH.
Material and Methods
CIH model
A total of 24 healthy 10-week-old male Wistar rats (220–250 g) were divided into 3 groups (n=8/group): the normoxia control group (CG), the intermittent hypoxia group (IH), and the TLR4 antagonist TAK242 treatment group (3 mg/kg, daily, dose chosen on the basis of previous studies reporting its anti-inflammatory role [18–21]), with exposure durations for 12 weeks and 16 weeks (HI) (these time periods were chosen based on a study of intermittent hypoxia simulating obstructive sleep apnea and causing pulmonary inflammation [17]). The IH groups rats were exposed to sham or chronic intermittent hypoxia (CIH) exposure. Rats were housed as normal in standard cages placed within commercially designed environmental chambers for daily gas treatments. A gas control system was used to regulate the flow of oxygen and nitrogen into the chamber. Ambient oxygen was servo-controlled to generate intermittent hypoxia. During a 2-min cycle, we first filled the chamber with nitrogen at a set rate to reach a fraction of inspired oxygen (FiO2) at 8% from 21% within 30 s. Then, compressed air was introduced into the chamber at a rate of 10 L/min to achieve a FiO2 of 21% within 50 s and compressed air at 5L/min was filled after that to maintain a level of 21% oxygen for 40 s until a new cycle began. Rats were placed into the chamber for 30 cycles/h for 8 h/day for 12 or 16 consecutive weeks. Rats in the control group were kept in the chamber with FiO2 of 21% throughout the experiment. The oxygen concentration in chambers was tested using a portable oxygen analyzer. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Shanxi Medical University.
Rats in the CH group received 3%O2+5%CO2+93%N2 while rats in the normal control group were only exposed to compressed air. An electrode was inserted into the cabin ventilation to confirm all changes in oxygen content. Oxygen content in the hypoxia cabin fluctuated between 8% and 21%, adjusted by the gas flow rate.
Drug treatment
The HI group rats were injected with TLR4-specific antagonist TAK242 daily 3 mg/kg for 12 or 16 weeks by intraperitoneal injection.
Tissues samples and hematoxylin and eosin staining (HE)
After 12 or 16 weeks, the rats were anesthetized. The anesthetized rat thorax was rapidly cut off with a surgical scissors, the chest cavity was opened, the surrounding tissue was isolated, the lung tissue was excised, the lung tissue was placed in a fixative solution of 4% paraformaldehyde+30% sucrose solution, embedded in paraffin, and cut into 10-um slices. Morphological changes in lung tissue were observed by HE staining. The dried slices were soaked in xylene, dewaxed 3 times for 10 min each, and then placed in100% and 95% alcohol 2 times for 2 min each time. The slices were washed with water then stained with hematoxylin for 5 min and washed 3 times, followed by a 1% hydrochloric acid alcohol differentiation for 1–2 s, 0.5% eosin staining for from 10 s to 1 min. Slices were then rinsed in 70% or 85% ethanol for 2 min each time and 95% ethanol for 3–5 s, then dried over anhydrous ethanol for 1 min for gradient alcohol dehydration, with final treatment 3 times with xylene for 2 min each time. Slices were removed and excess xylene was wiped off, then mounted with a drop of neutral gum. Finally, we used an optical microscope to observe changes in lung tissue morphology and structure at 100 magnification.
Immunohistochemistry analysis
Immunohistochemistry was used to detect the expression of TLR-4 in lung tissue. The slices were dehydrated in a grades series of ethanol concentrations for 8 min, 3% peroxide solution for 10 min, and distilled water 3 times for 2 min each. Then, the slices were placed in sodium citrate buffer at high temperature and pressure for 2 min, followed by washing in PBS 3 times for 2 min each time. We added 5% BSA liquid to stop the antigen reaction, followed by incubation for 20 min. We added TLR-4 antibody (concentration 1: 200), covering the lung tissue with antibody contact overnight at 4C. After rinsing in PBS, we added secondary antibody, followed by incubation at 37°C for 30 min and washing in PBS 3 times for 2 min each time. We then added SABC into the wet box for 20-min incubation, followed by washing in PBS 4 times. We added DAB for staining for 5 min to indicate the extent of distilled water rinse termination. Hematoxylin staining solution was added for 15 s, then tissue samples were rinsed in water for 5 min, then put in hydrochloric acid alcohol for differentiation. Tissue samples were examined under a microscope after gradient alcohol dehydration and being rendered transparent then mounted on slides. The results determine were deemed positive results if the cell membrane or cytoplasm was brown and negative if blue. ImagePlus4.0 software was used for semi-quantitative analysis of TLR-4 receptors in lung tissue according to the staining area and intensity.
Western blot
Whole tissue was lysed with RIPA-NP40 (KPL, USA) and tissue was separated on an 10% sodium dodecyl sulphate-(SDS-)polyacrylamide gel and blotted onto nitrocellulose membranes. The membranes were blocked with 5% BSA in TBST for 3 h at room temperature, then washed and incubated with primary anti-rat TLR-4 Abs (1/1000 dilution) (R&D Company, USA) overnight at 4°C. The membranes were washed with TBST and incubated with the appropriate secondary antibody (1/1000 dilution) (R&D Company, USA) for 1 h at room temperature. Detection of antigen was performed using the enhanced chemiluminescent detection method (Pierce, USA). The data were analyzed using Bio-Rad Image Lab densitometry software and normalized to β-actin bands.
Real-time fluorescence quantitative PCR
Real-time fluorescence quantitative PCR was also used to detect the expressions of TLR-4, MYD88, and NF-κB in lung tissues of rats. Primers were designed using Primer 3.0 software (http://frodo.wi.mit.edu/primer3). Total RNA was extracted from lung tissues of rats using Trizol reagent (Invitrogen, USA) following the manufacturer’s instructions. Relative mRNA expression levels were determined using the SYBR Green I kit (Biotechs, Changchun, China). Amplification was performed on the ABI Prism 7900HT Sequence Detection System (Applied Biosystems, California, USA) according to the following conditions: 1 cycle at 94°C for 2 min, 40 cycles at 94°C for 40 s, 60°C for 30 s, and 72°C for 30 s. Data are reported as values normalized by the housekeeping gene β-actin. Genetic relative quantitative was calculated with 2–ΔΔCt methods.
Enzyme-linked immunosorbent assay (ELISA)
The levels of TNF-α and IL-6 in lung tissue homogenate and serum were determined by ELISA. The rats were anesthetized, blood was drawn and centrifuged, supernatant was collected. We homogenized tissue lysate (0.5 mol/L NaCl, 50 mmol/L HEPES, 1% NP-40, and 1 g/L Leupeptin) to lyse lung tissue, and the homogenized supernatant was stored at −20°C for further use. ELISA was used to assess lung tissue homogenates and serum TNF-α and IL-10 levels according to kit instructions.
Oxidative stress index detection
Oxidative stress is also an important mechanism of CIH-induced lung injury. According to the manufacturer’s instructions, superoxide dismutase (SOD) and malondialdehyde (MDA) are commonly-used indicators of oxidative stress, lipid peroxidation, and subsequent cell damage. SOD and MDA kits were used to assess SOD and MDA levels in lung tissue samples.
Statistical analysis
All statistical analyses were carried out with SPSS21.0 software. Data are expressed as the mean ± standard deviation (χ̄±s). ANOVA was used to assess data from comparisons of multiple groups. Pairwise comparison among groups was undertaken using the LSD method, and correlation analysis was performed using the Pearson correlation test. P-values <0.05 were considered statistically significant.
Results
Establishment of the rat OSA model via IH
To determine the effect of OSAS on lung tissue, the rat OSAS model was established by performing IH for 12 weeks. The clinical severity of OSAS was time-dependent. Therefore, a 16-week OSHA rats model was established. To study the effect of TLR-4 on lung tissue damage caused by OSAS, the TLR-4-specific antagonist TAK242 was injected into rats in the hypoxic intervention group to observe changes in lung tissue injury.
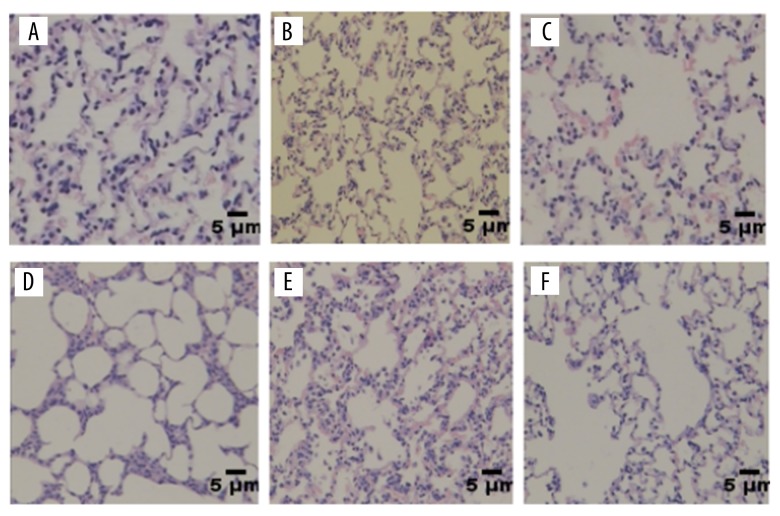
In the IH group, the alveolar area of the lung increased, and the alveolar septum thickened, fractured, and fused. In the IH group, the lung tissue injury was more severe in the 16-week group. The degree of lung injury in the rats injected with TLR242-TLR42 was reduced. In the normal control group, there was no significant change in lung alveolar area and alveolar septum (Figure 1A, 1B). In the IH 12-week group, the lung alveolar area slightly increased and the alveolar septum thickened and ruptured (Figure 1C). In the 16-week IH group, the degree of lung injury was more severe than that of the IH 12-week group (Figure 1D). The degree of destruction of lung tissue in the 12-week HI group was lower than that in the 12-week IH group (Figure 1E). The degree of lung destruction in the HI 16-week group was lower than that in the 16-week IH group (Figure 1F).
Figure 1.
Analysis of lung tissue hematoxylin and eosin staining. There was no significant change in lung alveolar area and alveolar septum in the normal control group (A, B). The alveolar area in lung tissue was slightly increased and the alveolar septum thickened and ruptured in the IH 12-week group (C). The lung tissue damage was more severe in the IH 16-week group (D). The degree of destruction of the lungs in the 12-week HI group was lower than in the 12-week IH group (E). The degree of lung destruction in the HI 16-week group was lower than in the IH 16-week group (F).
The IH group had an increase in alveolar mean liner spacing (MLI) and alveolar destruction index (DI) (p<0.05). The mean lining interval (MLI) and alveolar damage index (DI) in the 12-week IH group were higher than those in the normal control group and the HI group (P<0.05). The levels of MLI and DI were higher in the 16-week IH group than in the normal control group. There was a marked difference between the IH 12-week group and the HI group (P<0.05) (Table 1).
Table 1.
Comparision of MLI and DI in lung tissue of rats in each group (χ̄±s).
| Group | MLI (μm) | DI (%) |
|---|---|---|
| 12 W CG | 39.3526±2.0019 | 11.60±1.2974 |
| 16 W CG | 42.1744±2.8645* | 12.0608±2.0152* |
| 12 W IH group | 64.4819±1.4031* | 40.9635±3.6058* |
| 16 W IH group | 81.9350±4.9342# | 71.5750±6.0129# |
| 12 W HI group | 57.6064±6.8585*& | 29.1418±2.5066*& |
| 16 W HI group | 72.0778±3.7092#@ | 58.2440±2.9147#@ |
| F | 134.923 | 412.053 |
| P | 0.000 | 0.000 |
P <0.05 compared with 12W CG group;
P<0.05 compared with 16W CG group;
P<0.05 compared with 12W IH group;
P<0.05 compared with 16W IH group. n=8 samples/group.
OSAS increases the expression of TLR-4/MYD88/NF-κB in lung tissue
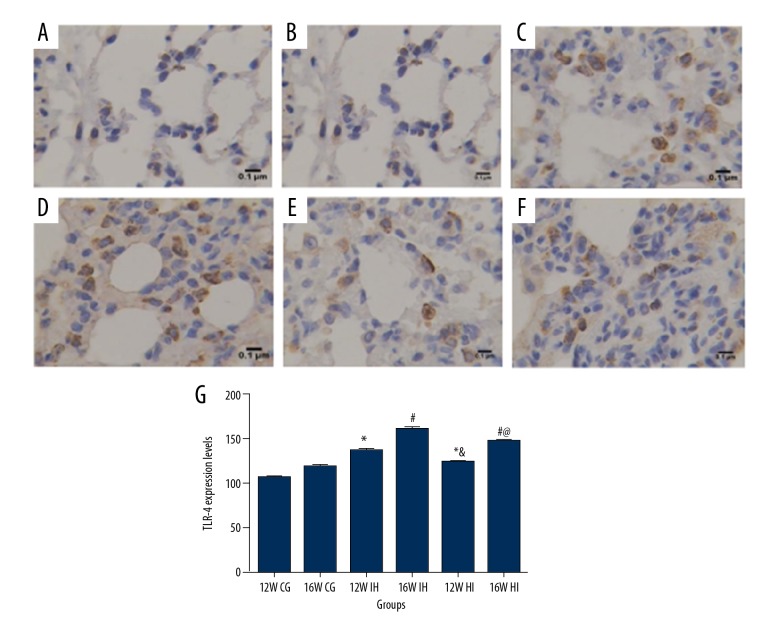
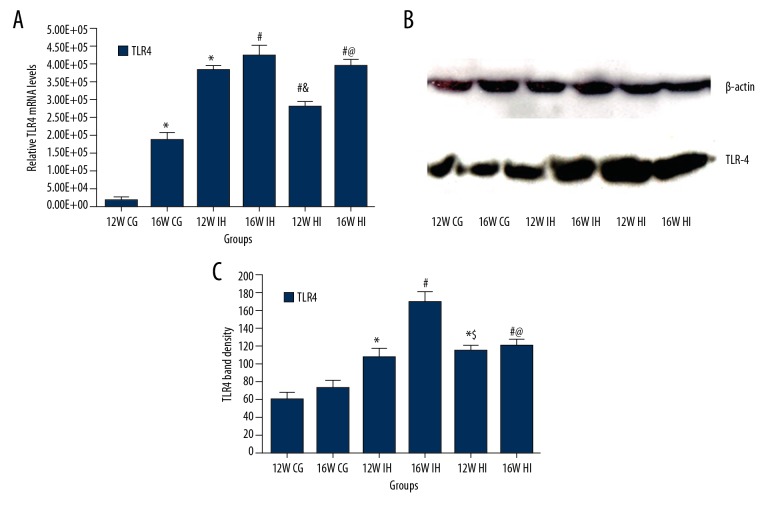
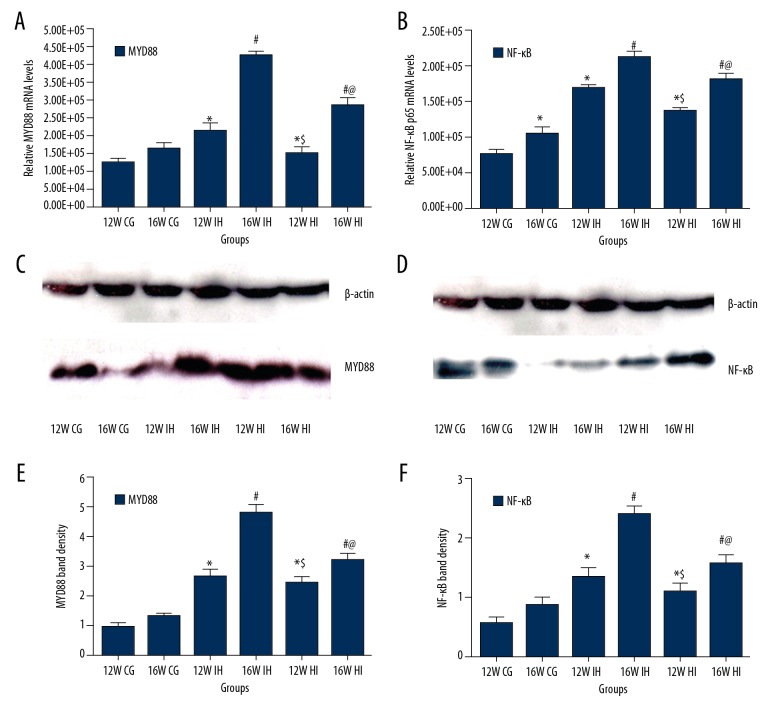
The expression of TLR-4/MYD88/NF-κB in lung tissue was tested by immunohistochemistry, Western blot, and PCR. Immunohistochemical staining showed that, compared with the control group (Figure 2A, 2B), the content of TLR-4 in the lung tissue of the IH group was higher (P<0.05) and it was associated with the IH increase (Figure 2C, 2D). The expression of TLR-4 in the HI group was higher than that in the control group but lower than that in the IH group (P<0.05; Figure 2E, 2F). The mRNA levels of TLR-4 (Figure 3A), MYD88 (Figure 4A), and NF-κB (Figure 4B) were detected by PCR. The results showed that the expression of TLR-4, MYD88, and NF-κB increased significantly in the intermittent hypoxia group compared with the normal group and the hypoxic intervention group, and these changes were IH time-dependent (p<0.05). Western blotting showed that the expression of TLR-4 (Figure 3B, 3C), MYD88 (Figure 4C, 4E), and NF-κB (Figure 4D, 4F) increased significantly in the intermittent hypoxia group compared with the normal group and hypoxic intervention group. (P<0.05).
Figure 2.
Immunohistochemical detection of TLR-4 expression in lung tissue, magnification ×400. (A) 12W CG group, (B) 16W CG group, (C) 12W IH group, (D) 16W IH group, (E) 12W HI group, and (F) 16W HI group. * Compared with 12W CG, P<0.05; # compared with 16W CG, P<0.05; compared with 12W IH group, P<0.05; @ compared with 16W IH group, P<0.05. n=8 samples/group.
Figure 3.
Real-time PCR results of mRNA levels of TLR-4 (A) and Western blotting results of expression of TLR-4 (B) tissues of rats in each group; TLR-4 band density (C). * P<0.05, compared to the 12W CG; # P<0.05, compared to the 16W CG; & P<0.05, compared to the 12W IH group; @ P<0.05, compared to the 16W IH group. n=8 samples/group.
Figure 4.
Relative gene expressions of MYD88 (A) and NF-κB (B) in lung tissues of rats. Relative protein expressions of MYD88 (C, E) and NF-κB (D, F) in the lung tissues of rats. The results were normalized with the housekeeping gene β-actin, and mRNA and protein levels in the normoxia group are presented as 100% (n=8/experimental group). * P<0.05, compared to the 12W CG; # P<0.05, compared to the 16W CG; & P<0.05, compared to the 12W IH group; @ P<0.05, compared to the 16W IH group. n=8 samples/group.
OSAS causes pulmonary inflammation and oxidative stress
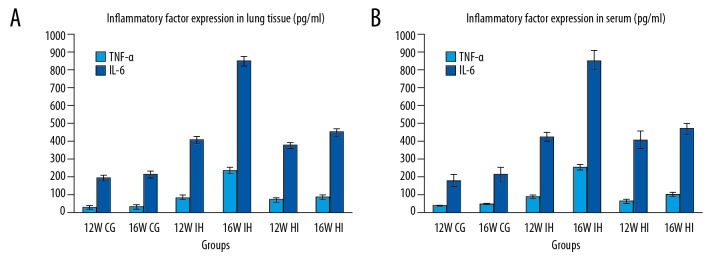
The expression of TNF-α and IL-6 in lung tissue (Figure 5A) and serum (Figure 5B) was detected by ELISA, showing that the content of TNF-α and IL-6 in lung tissue of the IH group was higher than that of normal control group and HI group (P<0.05). The content of TNF-α and IL-6 in the IH 16-week group was higher than that in the IH 12-week group (P<0.05).
Figure 5.
Expression levels of TNF-α (pg/ml) and IL-6 (pg/ml) in rat lung (A) and serum (B) (n=8/group), lung tissue and serum in IH group. The levels of TNF-α (pg/ml) and IL-6 (pg/ml) were significantly higher than those of the normal control group. The expression level of HI group was lower than that of the IH group but higher than that of the normal control group.
TAK242 impairs oxidative stress in lung tissue caused by IH
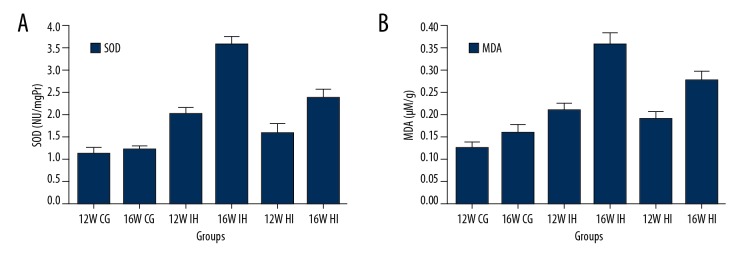
To assess the effect of TAK242 on oxidative stress in the IH model lung tissue, we measured the production of SOD (Figure 6A) and MDA (Figure 6B) in rat lung tissue. Compared with the normoxic group, the SOD and MDA contents in the lung tissue of the IH group were significantly increased, and TAK242 decreased the SOD and MDA levels in the lung tissue of the IH group.
Figure 6.
SOD (A) and MDA (B) in the lungs of rats (n=8 per group). SOD and MDA in the lung tissue in the IH group were significantly higher than those in the normal control group, while the levels in the HI group were lower than in the IH group but still higher than those in the IH group and normal control group.
Discussion
Obstructive sleep apnea syndrome (OSAS) is mainly characterized by the periodic or complete closure of the upper airway, usually with decreased oxygen saturation and repeated awakening. The incidence of OSAS is increasing steadily. Chronic intermittent hypoxia (CIH) is a hallmark feature of OSAS [22], in which humans, laboratory animals, and cells exhibit elevated levels of a variety of inflammatory cytokines that are associated with increased OSAS organ damage.
Chronic intermittent hypoxia is one of the most characteristic pathological changes in OSAS. This representative transient hypoxia-reoxygenation process is somewhat similar to ischemia-reperfusion injury [2]. Some studies have found that in this hypoxic/reoxygenation pathological process of ischemia-reperfusion, many reactive oxygen species (ROS) clusters are produced, which leads to the occurrence of oxidative stress in the body [4]. At the same time, with reactive oxygen species (ROS) increases in ROS and peroxides as second messengers initiate and regulate redox-sensitive signaling pathways and transcription factors such as nuclear factor NF-κB.
As an inducible and ubiquitous transcription factor, NF-κB can play a central regulatory role in many genes and plays an important role in immunization, inflammation, cell survival, proliferation, differentiation, and apoptosis. The nuclear factor NF-κB is closely related to the inflammatory response. There are 1 or more kB sequences in the promoters and enhancers of various inflammatory mediator genes, such as IL-1, IL-8, IL-6, TNF-alpha, VCAM-1, and ICAM-1, and activated NF-κB may participate in the induction of expression of the aforementioned media genes, either alone or in combination with other transcription factors [23].
TLR-4 is a type I transmembrane glycoprotein receptor that has an important role in the inflammatory response. The typical signaling pathway of TLR is the MyD88-dependent pathway, a major pathway on which almost all TLRs are dependent. After a series of phosphorylation and dephosphorylation, NF-κB and mitogen-activated protein kinases (MAPKs) are ultimately activated and induce the expression of inflammatory cytokines [24]. In recent years, it was found that the TLR-4/NF-κB signaling pathway is a signal transduction pathway closely related to inflammatory immune response, which is important in the development of inflammation.
Previous studies suggested that TLR-4, as an innate immune receptor, is often activated by bacterial lipopolysaccharide and triggers the corresponding inflammatory response. However, TLR-4/NF-κB activation may be attributed to intermittent hypoxia-induced ventricular remodeling. Local hypoxia and oxidative stress jointly mediate the damage and/or apoptosis of normal cells and release the molecular pattern of injury-related activation, thereby activating this inflammatory response pathway rather than the damage-related molecular pattern of bacterial inflammatory response, which activates the innate immune response. Akinnusi et al. [14] studied patients with OSAS and found that intermittent hypoxia time, monocyte TLR-4 surface, and cytoplasmic TLR-4 mRNA content increased, and found that NF-κB and its downstream corresponding inflammatory factors increased. This process is accomplished via the TLR-4/MYD88/NF-κB pathway.
A growing body of experimental evidence suggests that there is a nonbacterially active TLR4/NF-κB pathway involved in OSAS-induced central nervous system inflammation [25]. In the simulated OSAS pathology of our study rats, the lung tissue also showed different degrees of inflammatory response, showing that the TLR4/NF-κB inflammatory pathways may participate in this series of inflammatory reactions, and its expression in OSAS-induced lung injury and inflammation play a key role. With the development of OSAS, the process of rapid anoxia-reoxygenation during OSAS is somewhat similar to ischemia-reperfusion injury. Many recent studies showed that ischemia-reperfusion injury can activate the TLR-4/NF-κB inflammatory pathway [26–28]. There is evidence that TLR-4 has an adverse effect on ischemia-reperfusion injury after kidney transplantation [26], and the TLR-4/NF-κB pathway is closely related to myocardial ischemia-reperfusion injury [29]. The TLR4 pathway in cells protects the heart from ischemia-reperfusion injury [30]. Therefore, it is speculated that there is also a TLR-4/NF-κB inflammatory pathway in OSAS due to hypoxia-reoxygenation, a process similar to ischemia-reperfusion injury. Blocking the TLR-4 pathway can reduce CIH-induced lung injury and inflammation.
In our OSAS model, the inflammatory changes in lung tissue in rats were significantly changed, and the expressions of TLR-4, MYD88, p65, IL-6, TNF-α, SOD, and MDA were significantly increased compared to those in the normal control group. As the inhibition of TAK242 inhibitor lung tissue inflammation in rats in the lower-oxygen group was reduced, the above indicators were reduced. Therefore, we conclude that the TLR4/MYD88/NF-κB pathway is important in the lung injury and inflammatory response induced by CIH. However, TLR-4 antagonists do not completely protect lung tissue from CIH, and many studies show that target organ damage caused by CIH involves unwanted signal transduction pathways. It has been reported [31] that CIH is activated by the inflammatory pathways (NF-κB, TNF-α, and IL-6), the apoptotic pathway (BCL-2/Bax), and mitogen-activated protein kinases (phosphorylated P38, ERK, and JNK) signal transduction pathways to accelerate renal injury. Studies have shown [32] that CIH-induced elevation of blood pressure and aortic angiotensin II receptor type 1 (AT1R) expression plays an important role in hypertension, whereas increased IHC-induced AT1R expression may be mediated by regulation of p38MAPK and ERK1/2 and other different signal transduction pathways. It has been reported [33] that lovastatin achieves neuroprotection through modulation of NR2B-containing NMDA receptor-ERK pathway to attenuate learning and memory deficits caused by chronic intermittent hypoxemia-hypercapnia (CIHH). Therefore, it is unclear whether CIH can adversely affect lung tissue through other signaling pathways, and this topic warrants further research.
Conclusions
In summary, the chronic intermittent hypoxia-activated oxidative stress leads to a series of inflammatory responses and inflammatory cytokines release, resulting in lung injury and pulmonary inflammation. In our study the expression of TLR-4 in lung tissue was increased with prolonged hypoxia time. Furthermore, TLR-4-specific antagonists TAK242 reduced the pulmonary injury and inflammatory response induced by CIH. Therefore, we conclude that TLR-4 may play an important role in OSAS-induced lung inflammatory reaction, but whether there are other pathways involved needs to be confirmed by further studies.
Footnotes
Conflict of interest
None.
Source of support: This study was supported in part by funds from the International Cooperative Research and Development Project of Shanxi Science and Technology Department (No. 201703D421027) and the Shanxi Province Human Resources and Social Development Department for Returned Chinese Scholars (2017-2011008-302)
References
- 1.Jia W, Jian Z, Li J, et al. Upregulated ATF6 contributes to chronic intermittent hypoxia-afforded protection against myocardial ischemia/reperfusion injury. Int J Mol Med. 2016;37(5):1199–208. doi: 10.3892/ijmm.2016.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Passali D, Corallo G, YAREMCHUK S, et al. Oxidative stress in patients with obstructive sleep apnea syndrome. Acta Otorhinolaryngol Ital. 2015;35(6):420–25. doi: 10.14639/0392-100X-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Q, Du J, Ling X, Lu Y. Evaluation of MIh scoring system in diagnosis of obstructive sleep apnea syndrome. Med Sci Monit. 2017;23:4715–22. doi: 10.12659/MSM.904087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, Zheng L, Cao J, et al. Inflammation induced by increased frequency of intermittent hypoxia is attenuated by tempol administration. Braz J Med Biol Res. 2015;48(12):1115–21. doi: 10.1590/1414-431X20154487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baud V, Collares D. Post-translational modifications of RelB NF-κB subunit and associated functions. Cells. 2016;5(2):22. doi: 10.3390/cells5020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Israel LP, Benharoch D, Gopas J, Goldbart AD. A pro-inflammatory role for nuclear factor kappa B in childhood obstructive sleep apnea syndrome. Sleep. 2013;36(12):1947–55. doi: 10.5665/sleep.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei Q, Bian Y, Yu F, et al. Chronic intermittent hypoxia induces cardiac inflammation and dysfunction in a rat obstructive sleep apnea model. J Biomed Res. 2016;30(6):490–95. doi: 10.7555/JBR.30.20160110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prabhakar NR, Semenza GL. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol Rev. 2012;92(3):967–1003. doi: 10.1152/physrev.00030.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maglione PJ, Simchoni N, Cunningham-Rundles C. Toll-like receptor signaling in primary immune deficiencies. Ann NY Acad Sci. 2015;1356(1):1–21. doi: 10.1111/nyas.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, He H, Li D, et al. The role of the TLR4 signaling pathway in cognitive deficits following surgery in aged rats. Mol Med Rep. 2013;7(4):1137–42. doi: 10.3892/mmr.2013.1322. [DOI] [PubMed] [Google Scholar]
- 11.De Nardo D. Toll-like receptors: Activation, signalling and transcriptional modulation. Cytokin. 2015;74(2):181–89. doi: 10.1016/j.cyto.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 12.Chen D, Pan D, Tang S, et al. Administration of chlorogenic acid alleviates spinal cord injury via TLR4/NF κB and p38 signaling pathway anti inflammatory activity. Mol Med Rep. 2018;17(1):1340–46. doi: 10.3892/mmr.2017.7987. [DOI] [PubMed] [Google Scholar]
- 13.Zhong K. Curcumin mediates a protective effect via TLR-4/NF-κB signaling pathway in rat model of severe acute pancreatitis. Cell Biochem Biophys. 2015;73(1):175–80. doi: 10.1007/s12013-015-0664-y. [DOI] [PubMed] [Google Scholar]
- 14.Akinnusi M, Jaoude P, Kufel T, El-Solh AA. Toll-like receptor activity in patients with obstructive sleep apnea. Sleep Breath. 2013;17(3):1009–16. doi: 10.1007/s11325-012-0791-2. [DOI] [PubMed] [Google Scholar]
- 15.Poulain L, Richard V, Lévy P, et al. Toll-like receptor-4 mediated inflammation is involved in the cardiometabolic alterations induced by intermittent hypoxia. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/620258. 620258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merry HE, Phelan P, Doak MR, et al. Role of toll-like receptor-4 in lung ischemia-reperfusion injury. Ann Thorac Surg. 2015;99(4):1193–99. doi: 10.1016/j.athoracsur.2014.12.062. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Chai Y, He X, et al. Intermittent hypoxia simulating obstructive sleep apnea causes pulmonary inflammation and activates the Nrf2/HO-1 pathway. Exp Ther Med. 2017;14(4):3463–70. doi: 10.3892/etm.2017.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sha T, Iizawa Y, Ii M. Combination of imipenem and TAK-242, a Toll-like receptor 4 signal transduction inhibitor, improves survival in a murine model of polymicrobial sepsis. Shock. 2011;35:205–9. doi: 10.1097/SHK.0b013e3181f48942. [DOI] [PubMed] [Google Scholar]
- 19.Yao L, Kan EM, Lu J, et al. Toll-like receptor 4 mediates microglial activation and production of inflammatory mediators in neonatal rat brain following hypoxia: Role of TLR4 in hypoxic microglia. J Neuroinflammation. 2013;10:23. doi: 10.1186/1742-2094-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Tatsui CE, Rhines LD, et al. Dorsal root ganglion neurons become hyperexcitable and increase expression of voltage-gated T-type calcium channels (Cav3.2) in paclitaxel-induced peripheral neuropathy. Pain. 2017;158(3):417–29. doi: 10.1097/j.pain.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Peng W, Ao X, et al. TAK-242, a toll-like receptor 4 antagonist, protects against aldosterone-induced cardiac and renal injury. PLoS One. 2015;10(11):e0142456. doi: 10.1371/journal.pone.0142456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou YQ, Ye JY. [Personalized combined modality therapy based on pathophysiology of obstructive sleep apneahypopnea syndrome]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016;51(11):877–80. doi: 10.3760/cma.j.issn.1673-0860.2016.11.020. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 23.Pal S, Bhattacharjee A, Ali A, et al. Chronic inflammation and cancer: Potential chemoprevention through nuclear factor kappa B and p53 mutual antagonism. J Inflamm (Lond) 2014;11:23. doi: 10.1186/1476-9255-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vallance TM, Zeuner MT, Williams HF, et al. Toll-like receptor 4 signaling and its impact on platelet function, thrombosis, and haemostasis. Mediators Inflamm. 2017;2017 doi: 10.1155/2017/9605894. 9605894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Q, Wang Y, Feng J, et al. Intermittent hypoxia from obstructive sleep apnea may cause neuronal impairment and dysfunction in central nervous system: The potential roles played by microglia. Neuropsychiatr Dis Treat. 2013;9:1077–86. doi: 10.2147/NDT.S49868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao H, Perez JS, Lu K, et al. Role of Toll-like receptor-4 in renal graft ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2014;306(8):F801–11. doi: 10.1152/ajprenal.00469.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishizuka F, Shimazawa M, Inoue Y, et al. Toll-like receptor 4 mediates retinal ischemia/reperfusion injury through nuclear factor-κB and spleen tyrosine kinase activation. Invest Ophthalmol Vis Sci. 2013;54(8):5807–16. doi: 10.1167/iovs.13-11932. [DOI] [PubMed] [Google Scholar]
- 28.Phelan P, Merry HE, Hwang B, Mulligan MS. Differential Toll-like receptor activation in lung ischemia reperfusion injury. J Thorac Cardiovasc Surg. 2015;149(6):1653–61. doi: 10.1016/j.jtcvs.2015.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen H, Zhang RQ, Wei XG, et al. Mechanism of TLR-4/NF-κB pathway in myocardial ischemia reperfusion injury of mouse. Asian Pac J Trop Med. 2016;9(5):503–7. doi: 10.1016/j.apjtm.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 30.Vicencio JM, Yellon DM, Sivaraman V, et al. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J Am Coll Cardiol. 2015;65(15):1525–36. doi: 10.1016/j.jacc.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 31.Wu X, Gu W, Lu H, et al. Soluble receptor for advanced glycation end product ameliorates chronic intermittent hypoxia induced renal injury, inflammation, and apoptosis via P38/JNK signaling pathways. Oxid Med Cell Longev. 2016;2016 doi: 10.1155/2016/1015390. 1015390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shang J, Yang YY, Guo XL, Liu HG. Ang II type 1 receptor expression in rat aorta exposed to chronic intermittent hypoxia: Effects of p38MAPK and ERK1/2 signaling. Chin Med J (Engl) 2013;126(17):3264–69. [PubMed] [Google Scholar]
- 33.Huo XL, Min JJ, Pan CY. Efficacy of lovastatin on learning and memory deficits caused by chronic intermittent hypoxia-hypercapnia: through regulation of NR2B-containing NMDA receptor-ERK pathway. PLoS One. 2014;9(4):e94278. doi: 10.1371/journal.pone.0094278. [DOI] [PMC free article] [PubMed] [Google Scholar]