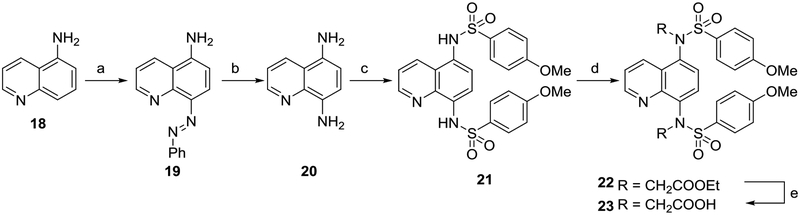
Scheme 3. Synthesis of quinolines 21 and 23a.
aReagents: (a) aniline, NaNO2, HCl, AcOH, NaOAc, H2O, 30% yield; (b) SnCl2, HCl, reflux, 50% yield; (c) p-methoxybenzenesulfonyl chloride, pyridine, rt, 77% yield; (d) RBr, K2CO3, DMF, rt; (e) NaOH, MeOH, reflux, 67% yield over 2 steps. rt: room temperature