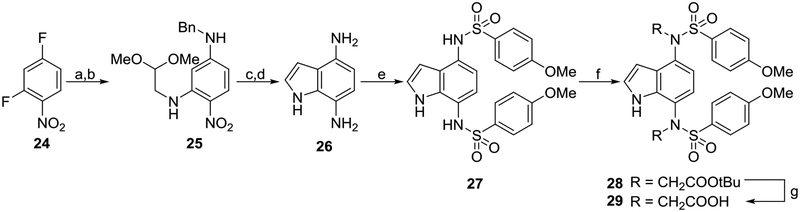
Scheme 4. Synthesis of indoles 27 and 29a.
aReagents: (a) aminoacetaldehyde dimethyl acetal, i-Pr2NEt, MeOH, reflux, 86% yield; (b) benzylamine, 1,4-dioxane, reflux, 93% yield; (c) F3CCO2H, CH2Cl2, rt, 85% yield; (d) Pd/C, EtOH/HCl, H2, 35 psi, 100% yield; (e) p-methoxybenzenesulfonyl chloride, pyridine, rt, 60% yield; (f) RBr, K2CO3, DMF, rt, 82% yield; (g) TFA, CH2Cl2, rt, 20% yield. rt: room temperature