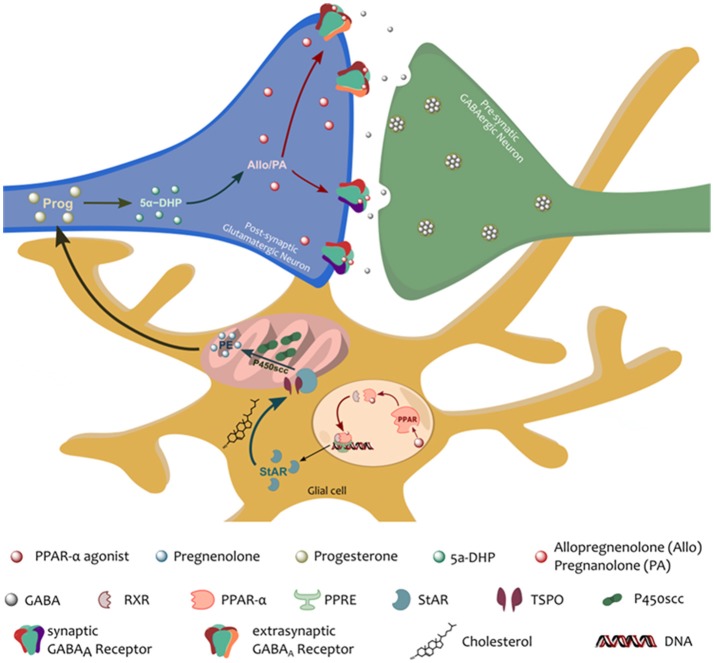
Figure 1.
Schematic representation of the proposed PPAR–α-allopregnanolone biomarker axis. PPAR–α, following its activation by an endogenous (e.g., PEA) or a synthetic agonist, heterodimerizes with a PPAR–α-specific retinoid X receptor (RXR). The PPAR-RXR dimer then binds the PPAR response element (PPRE) in specific promoter regions that up- or down-regulate gene expression. PPAR–α activation would therefore normalize the stress-induced downregulation of neurosteroidogenic proteins, StAR and p450scc. StAR, is crucial to the translocation of cholesterol into the inner mitochondrial membrane. There, cholesterol is metabolized by the action of the P450scc into pregnenolone (the precursors of all neurosteroids). Pregnenolone can be further converted to progesterone and 5α-dihydroprogesterone (5α-DHP) by the action of 5α-reductase type I. 5α-DHP can then be converted by 3α-hydroxysteroid dehydrogenase into allopregnanolone (Allo) and its equipotent isomer, pregnanolone (PA), which allows for potent, positive, allosteric potentiation of the GABAA receptors located in the post-synaptic membrane of pyramidal neurons of the frontal cortex and hippocampus, and pyramidal-like neurons of the basolateral amygdala (Agís-Balboa et al., 2006, 2007; Pinna et al., 2008).