Abstract
Background
Surgical-site infection (SSI) is associated with significant healthcare costs. To reduce the high rate of SSI among patients undergoing colorectal surgery at a cancer centre, a comprehensive care bundle was implemented and its efficacy tested.
Methods
A pragmatic study involving three phases (baseline, implementation and sustainability) was conducted on patients treated consecutively between 2013 and 2016. The intervention included 13 components related to: bowel preparation; oral and intravenous antibiotic selection and administration; skin preparation, disinfection and hygiene; maintenance of normothermia during surgery; and use of clean instruments for closure. SSI risk was evaluated by means of a preoperative calculator, and effectiveness was assessed using interrupted time-series regression.
Results
In a population with a mean BMI of 30 kg/m2, diabetes mellitus in 17·5 per cent, and smoking history in 49·3 per cent, SSI rates declined from 11·0 to 4·1 per cent following implementation of the intervention bundle (P = 0·001). The greatest reductions in SSI rates occurred in patients at intermediate or high risk of SSI: from 10·3 to 4·7 per cent (P = 0·006) and from 19 to 2 per cent (P < 0·001) respectively. Wound care modifications were very different in the implementation phase (43·2 versus 24·9 per cent baseline), including use of an overlying surface vacuum dressing (17·2 from 1·4 per cent baseline) or leaving wounds partially open (13·2 from 6·7 per cent baseline). As a result, the biggest difference was in wound-related rather than organ-space SSI. The median length of hospital stay decreased from 7 (i.q.r. 5–10) to 6 (5–9) days (P = 0·002). The greatest reduction in hospital stay was seen in patients at high risk of SSI: from 8 to 6 days (P < 0·001). SSI rates remained low (4·5 per cent) in the sustainability phase.
Conclusion
Meaningful reductions in SSI can be achieved by implementing a multidisciplinary care bundle at a hospital-wide level.
Protocol-driven programme works
Introduction
Surgical-site infection (SSI) accounts for more than one-third of all inpatient infectious events1. SSI can manifest as wound erythema and discharge when it is superficial, or as sepsis when it is deep and involves fascia or an intra-abdominal organ space. Along with added morbidity and delayed convalescence, SSI places considerable financial strain on the healthcare system2,3 owing to prolonged hospital care, readmission and disability4–6. Treatments include opening a surgical incision, antibiotics, or an invasive procedure to drain an abscess or debride tissue. A perioperative mortality rate for SSI of 3 per cent has been reported, with 75 per cent of associated deaths directly attributable to the SSI7.
SSI is a direct consequence of surgery, and some instances may be preventable. Intensive quality improvement initiatives have been developed in the USA, including the Surgical Infection Prevention Project8 and the Surgical Care Improvement Project (SCIP)9. These initiatives were first implemented for high-risk procedures such as colorectal surgery6. The aim of the SCIP was to improve processes of care by having hospitals follow best practices, including appropriate administration of antibiotics, use of optimal hair removal techniques and maintenance of normothermia. However, adoption of SCIP best practice measures did not uniformly lead to SSI reduction10–13. Experts have argued that more comprehensive programmes that inspire healthcare providers to develop and implement solutions along with cultural change are necessary to achieve meaningful reduction in preventable healthcare-associated complications14–21. Model programmes include those aimed at reducing central-line bloodstream infections22, ventilator-associated pneumonias23 and catheter-associated urinary tract infections24. This pragmatic study was designed to test the efficacy of a care bundle in reducing SSI at the authors' centre.
Methods
Development of the surgical-site infection reduction programme
In 2013, Memorial Sloan Kettering Cancer Center (MSK), a National Cancer Institute-designated comprehensive cancer centre, developed an SSI reduction programme. Representatives from the Departments of Surgery, Medicine, Anaesthesia, Nursing, Administration, Infection Control, and Quality and Safety were assembled to review care of surgical patients along the spectrum from preoperative evaluation to discharge from the hospital. The literature for optimal preoperative, intraoperative and postoperative care was reviewed as described elsewhere15,21, and practices were chosen on the basis of high levels of supporting evidence or their being considered (by consensus of multidisciplinary team representatives) reasonable, associated with minimal risk and potentially beneficial. In addition to SCIP measures related to preoperative administration and postoperative discontinuation of antibiotics that were instituted before 2013, a number of standard procedures and approaches were newly implemented (Table 1).
Table 1.
Components of the surgical-site infection reduction bundle
| Compliance (%) | |||||
|---|---|---|---|---|---|
| Source | Enactment team* | Measurement method | Baseline phase | Implementation phase | |
| Preoperative | |||||
| Appropriate antibiotic selection | SCIP | PAT nursing | EMR | 99·6 | 99·4 |
| Consultation for raised haemoglobin A1C level | MDT | PAT nursing | EMR | 90·3 | |
| Chlorhexidine shower | MDT | OC nursing | PPQ | ||
| Night before surgery | 91·6 | ||||
| Morning of surgery | 96·1 | ||||
| Mechanical bowel preparation | MDT | OC nursing | PPQ | 91·2 | |
| Oral antibiotics | MDT | OC nursing | PPQ | ||
| Early evening | 93·0 | ||||
| Late evening | 88·0 | ||||
| SSI risk assessment provided to surgeon | MDT | QA | n.a. | 100 | |
| Intraoperative | |||||
| Antibiotic administration before incision | SCIP | Anaesthesia | EMR | 99·4 | 98·9 |
| Appropriate method of hair removal | SCIP | Surgery | EMR | 100 | 100 |
| Maintenance of normothermia† | SCIP | Anaesthesia | EMR | 100 | 100 |
| Intraoperative antibiotic redosing | MDT | Anaesthesia | EMR | 85·3 | |
| Closing tray for open procedures | MDT | Surgery | n.a. | 100 | |
| Postoperative | |||||
| Discontinuation of antibiotics at 24 h | SCIP | Surgery | EMR | 100 | 100 |
| Shower on postoperative day 2 | MDT | IP nursing | n.a. | ||
Data are reported in accordance with the CONSORT guidelines for pragmatic trials25.
Nursing includes registered nurses and nurse practitioners; surgery includes attending surgeons, residents and physician assistants.
Temperature measured on arrival at the postanaesthesia care unit.
SCIP, Surgical Care Improvement Project; PAT, preadmission testing unit; EMR, electronic medical record; MDT, Memorial Sloan Kettering multidisciplinary team; OC, outpatient clinic; PPQ, preoperative patient questionnaire; SSI, surgical-site infection; QA, quality assurance; n.a., not applicable; IP, inpatient.
A unique intervention that was included comprised providing surgeons with an estimated risk of SSI, via e-mail the day before surgery. It was hypothesized that this information might influence surgeons' decisions regarding wound care and method of wound closure. For example, partial skin closure, subcutaneous drains and negative-pressure surface vacuum dressings on closed incisions might reduce superficial SSI26. Any method of closure other than primary closure with a dry dressing was considered ‘modified’.
Initially, the National Nosocomial Infections Surveillance (NNIS) risk index, developed by the Centers for Disease Control and Prevention (CDC), was evaluated as described by the CDC27 on a historical population of 1471 patients who underwent colorectal resection during calendar years 2011 and 2012 at MSK (Table S1, supporting information). Preliminary analysis revealed that the model had modest predictive performance, with a concordance index of 0·64. Using this data set, with an overall SSI rate of 13·0 per cent, a custom, colorectal surgery-specific SSI prediction tool was developed. The goal was to develop an automated tool using variables from the electronic medical record. Five clinically relevant factors associated with SSI were chosen for the MSK colorectal SSI prediction tool: concurrent liver surgery, history of smoking, duration of surgery, Charlson Co-morbidity Index and BMI (Appendix S1, supporting information). In logistic regression modelling, the tool's area under the curve/concordance index for predicting SSI was 0·74.
The intervention began on 1 November 2013, with adoption of all 13 components of the SSI reduction programme. Compliance with the SSI reduction measures was audited regularly by automatic medical record review and with a patient questionnaire completed on the day of surgery (Table 1). Non-compliance triggered a chart review, and compliance data were reviewed at monthly multidisciplinary SSI meetings.
Design of the pragmatic study
Implementation of the bundle precluded randomization. To minimize biases in the absence of random treatment assignment, the study consisted of three phases: baseline, implementation and sustainability24. The existing MSK SSI tracking programme was used, which had been implemented to comply with the New York State law mandating that all patients undergoing colorectal surgery be monitored for 30 days and SSI reported to the state every month.
SSI was defined according to CDC guidelines28. Superficial SSI involves skin and subcutaneous tissue, deep SSI involves fascia and muscle, and organ-space SSI involves the intra-abdominal space. Because precise distinction of superficial from deep SSI can be a challenge, SSI is categorized in the institutional database as superficial/deep or organ space. Disease and procedure codes used as inclusion criteria are listed in Appendix S1 (supporting information). As all eligible patients were included in the pragmatic design, informed consent was not obtained. The study was conducted as a performance improvement project in concordance with institutional review board policy.
To be meaningful and important, a relative reduction in the rate of SSI following implementation of the SSI reduction programme would have to be at least 50 per cent. With an anticipated SSI rate of 10 per cent in the baseline phase, 450 to 500 patients in each study arm would allow more than 80 per cent power to detect an SSI rate reduction to 5 per cent, controlling the type I error rate at 5 per cent, using the χ2 test. Interrupted time-series analysis was employed to rule out the possibility that any changes in SSI rate from baseline to intervention were the consequence of time trends29.
Using surgical volume projections, the baseline phase of the study included patients who underwent surgery between 1 January and 31 October 2013. The SSI reduction programme went into effect on 1 November 2013, and the implementation phase of the study included patients who underwent surgery between 1 November 2013 and 31 December 2014. The sustainability phase included patients who had surgery between 1 January 2015 and 31 March 2016.
In addition to providing an estimated risk of SSI to surgeons before surgery, the SSI prediction tool was used to group patients by SSI risk level. Low-, intermediate- and high-risk groups were defined as groups of patients for whom the risk of SSI was below 0·07, between 0·07 and 0·21, and over 0·21 respectively, according to the MSK prediction tool.
Results
The baseline phase of the trial included 454 patients, the implementation phase 616 patients and the sustainability phase 758 patients. Clinical characteristics and types of surgery for the baseline and implementation phase are listed in Table 2. Compliance with SCIP guidelines (monitored at MSK since 2007) was over 98 per cent in both the baseline and implementation phases; compliance in the implementation phase with SSI reduction practices instituted in 2013 was at least 85 per cent (Table 1).
Table 2.
Characteristics of patients and treatments in baseline and implementation phases
| Baseline phase (n = 454) | Implementation phase (n = 616) | P ‡ | |
|---|---|---|---|
| Age (years)* | 61(14) | 61(14) | 0·817§ |
| Sex ratio (F : M) | 236 : 218 | 316 : 300 | 0·853 |
| BMI (kg/m2)* | 30(6) | 30(6) | 1·000§ |
| History of diabetes | 79 (17·4) | 108 (17·5) | 1·000 |
| History of smoking | 222 (48·9) | 306 (49·7) | 0·805 |
| CCI score* | 9·0(3·2) | 8·8(3·3) | 0·219§ |
| ASA fitness grade III | 339 (74·7) | 471 (76·5) | 0·517 |
| Wound class clean-contaminated | 364 (80·2) | 495 (80·4) | 1·000 |
| Duration of operation (min)† | 240 (57–945) | 259 (66–825) | 0·427§ |
| Surgical procedure | |||
| Resection type | 0·377 | ||
| Right colectomy | 227 (50·0) | 306 (49·7) | |
| Left colectomy | 51 (11·2) | 66 (10·7) | |
| Sigmoid colectomy | 55 (12·1) | 71 (11·5) | |
| Total colectomy | 27 (5·9) | 40 (6·5) | |
| Rectal resection | 51 (11·2) | 52 (8·4) | |
| Other colorectal | 43 (9·5) | 81 (13·1) | |
| Combined colorectal and liver | 26 (5·7) | 27 (4·4) | 0·322 |
| Open | 212 (46·7) | 348 (56·5) | 0·002 |
Values in parentheses are percentages unless indicated otherwise;
values are mean(s.d.) and
median (range).
CCI, Charlson Co-morbidity Index.
Fisher's exact test, except
Wilcoxon test.
Surgical-site infection rates
The SSI rate in the implementation phase was significantly lower than that in the baseline phase (4·1 versus 11·0 per cent; P = 0·001) (Table 3). Interrupted time-series analysis (Fig. 1) did not reveal any significant time trends in the baseline phase (P = 0·358) or implementation phase (P = 0·441), suggesting the reduction in SSI was the result of the intervention.
Table 3.
Surgical-site infection rates
| Surgical-site infection | P * | ||
|---|---|---|---|
| Baseline phase (n = 454) | Implementation phase (n = 616) | ||
| Superficial/deep | 36 (7·9) | 11 (1·8) | < 0·001 |
| Organ space | 14 (3·1) | 14 (2·3) | 0·219 |
| Total | 50 (11·0) | 25 (4·1) | 0·001 |
Values in parentheses are percentages.
Fisher's exact test.
Fig. 1.
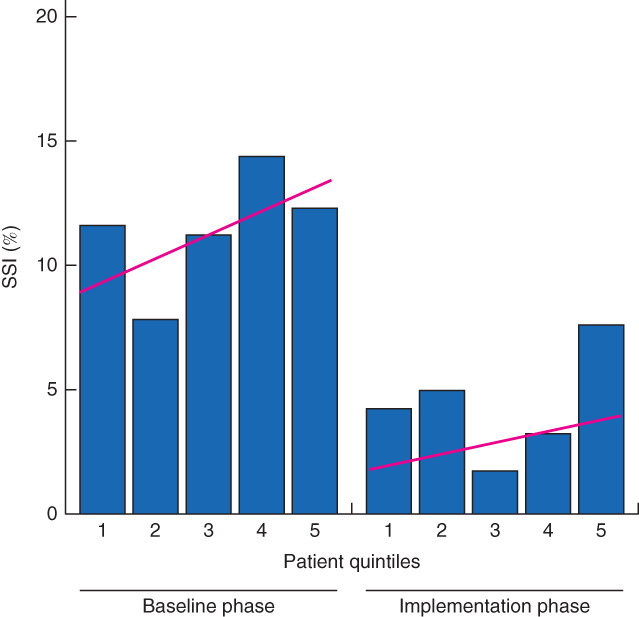
Surgical-site infection (SSI) rates over time. Each bar represents 20 per cent of consecutive surgical patients in the corresponding study phase. Estimated time trends are indicated
SSI rates for the three risk groups are shown in Table 4. The intermediate-risk group in the implementation phase had a significantly lower SSI rate than in the baseline phase (4·7 versus 10·3 per cent; P = 0·006). Likewise, the high-risk group in the implementation phase had a significantly lower SSI rate than in the baseline phase (2 versus 19 per cent; P < 0·001).
Table 4.
Surgical-site infection rates by infection risk group
| Surgical-site infection | P * | ||
|---|---|---|---|
| Baseline phase | Implementation phase | ||
| Low-risk group | n = 93 | n = 122 | |
| Superficial/deep | 4 (4) | 0 (0) | 0·001 |
| Organ space | 2 (2) | 4 (3·3) | 0·700 |
| Total | 6 (6) | 4 (3·3) | 0·334 |
| Intermediate-risk group | n = 282 | n = 408 | |
| Superficial/deep | 22 (7·8) | 10 (2·5) | 0·034 |
| Organ space | 7 (2·5) | 9 (2·2) | 0·803 |
| Total | 29 (10·3) | 19 (4·7) | 0·006 |
| High-risk group | n = 79 | n = 86 | |
| Superficial/deep | 10 (13) | 1 (1) | 0·004 |
| Organ space | 5 (6) | 1 (1) | 0·087 |
| Total | 15 (19) | 2 (2) | < 0·001 |
Values in parentheses are percentages.
Fisher's exact test.
When analysed by type of infection, differences in the rates of superficial/deep SSIs between the baseline and implementation phases were statistically significant for all three risk groups (P = 0·001, P = 0·034 and P = 0·004 for low-, intermediate- and high-risk groups respectively), but the differences in organ-space SSI were not statistically significant.
Wound closure
In the implementation phase, wound closure modifications were used in a larger proportion of patients than in the baseline phase (43·2 versus 24·9 per cent respectively). In the baseline phase, incisions were closed primarily with a dry dressing in 75·1 per cent, closed over a drain in 16·8 per cent, closed with an overlying surface vacuum dressing in 1·4 per cent and left partially open in 6·7 per cent. In the implementation phase, incisions were closed primarily with a dry dressing in 57·0 per cent, closed over a drain in 12·6 per cent, closed with an overlying surface vacuum dressing in 17·2 per cent and left partially open in 13·2 per cent. For the low-, intermediate- and high-risk groups, wound closure modifications were used in 14, 22·2 and 39 per cent of patients respectively in the baseline phase, compared with 27·7, 42·3 and 57 per cent in the implementation phase.
Length of hospital stay
The median length of hospital stay (LOS) was significantly shorter in the implementation phase than in the baseline phase: 6 (i.q.r. 5–9) versus 7 (5–10) days respectively (P = 0·002). For the intermediate-risk group, median LOS was significantly lower in the implementation phase than in the baseline phase: 6 (5–8) versus 7 (5–10) days (P = 0·006). Likewise, for the high-risk group, median LOS was significantly lower in the implementation phase than in the baseline phase: 6 (5–9) versus 8 (6–12) days (P < 0·001).
Median LOS was significantly lower in patients without SSI than among those with SSI in both the baseline and implementation phases. In the baseline phase, the median LOS was 7 (5–10) days for patients without SSI and 8·5 (6–14) days for patients with SSI (P = 0·016). Respective values in the implementation phase were 6 (5–8) and 8 (6–27) days (P = 0·001).
Readmission rates
The 30-day readmission rate was 14·1 per cent in the baseline phase and 11·7 per cent in the implementation phase (P = 0·265). It was significantly lower for patients without SSI than for patients with SSI in both the baseline phase (10·7 versus 42 per cent; P < 0·001) and the implementation phase (10·2 versus 46 per cent; P < 0·001).
Analysis of surgical-site infection in the implementation phase
In the implementation phase, the 25 patients with SSI had a significantly higher rate of concurrent liver surgery (5 versus 0 per cent; P < 0·001) and a somewhat higher rate of diabetes (19 versus 7·7 per cent; P = 0·058) than the 591 patients without SSI. There was no difference between patients with SSI and those without in terms of BMI, smoking history, Charlson Co-morbidity Index, rate of laparoscopic procedures, or compliance with the SSI reduction bundle.
Sustainability phase
In the sustainability phase, 34 of the 758 patients had an SSI, giving a rate of 4·5 (95 per cent c.i. 3·1 to 6·2) per cent, indicating that the SSI reduction programme had a sustained impact.
Discussion
The multidisciplinary care bundle reported here significantly reduced the SSI rate at MSK. The greatest declines occurred in the rates of superficial/deep SSIs (as defined by the CDC), which involve the skin and/or fascia, in agreement with other reports15,30. Organ-space SSIs were not affected, which is not surprising as such infections usually result from anastomotic dehiscence or leak and are minimally influenced by interventions included in the patient care bundle. Implementation of the care bundle reduced LOS but not the 30-day readmission rate, probably because superficial and deep wound infections can be treated adequately on an outpatient basis, whereas more significant SSI requires readmission.
The observed reduction in SSI rate likely resulted from implementation of the bundle, as patient characteristics and risk levels did not change over time, other than a slight increase in open procedures in the implementation phase. The median risk level in the implementation phase did not differ from that in the baseline phase, indicating that the decline in SSI was not a result of a higher proportion of low-risk patients in the implementation phase. In addition, interrupted time-series analysis (Fig. 1) demonstrated that there were no time trends to explain the reduction in SSI. This supports the conclusion that the reduction in SSI is attributed to implementation of the intervention.
Previously reported multidisciplinary interventions aimed at reducing SSI rates achieved various degrees of success15,18,21 and failure13,31. A recent meta-analysis32 of 13 trials with 8515 patients found that implementation of a surgical bundle in patients undergoing colorectal surgery significantly reduced the SSI rate, although there was variation in bundle components and success.
The present intervention differs from others in that it involves using the MSK colorectal SSI prediction tool to inform surgeons of the SSI risk before surgery, which may influence how a surgeon manages a patient's treatment. The prediction tool also facilitates grouping of patients by SSI risk and comparison of groups with a similar risk. The MSK colorectal SSI prediction tool was created because the NNIS model did not perform well in MSK's cancer-based practice. The difficulty in applying risk calculators developed in one population to another population was described recently by Bergquist and colleagues33, who evaluated a variety of SSI risk scoring systems in a series of 2376 patients at a single institution. The authors reported concordance indices ranging from 0·57 to 0·62, and hypothesized that institution- and case mix-specific factors accounted for the poor results. Similarly, the NNIS model was not sufficient for the colorectal cancer population at MSK, which has a relatively high rate of concurrent liver resections, with a concordance index of 0·64. The concordance index of the MSK colorectal SSI risk model was 0·74, well within the expected range for a prognostic biological model.
The MSK colorectal SSI prediction tool provided surgeons with an assessment of the risk of SSI before surgery, which may have influenced the method of wound closure in higher-risk patients. In the baseline phase, wound closure modifications26 were employed in one-quarter of all patients, with a 73 per cent relative increase seen in the implementation phase. The greatest increase was noted for low- and intermediate-risk patients. Wound closure modifications were used in over 40 per cent of high-risk patients in the baseline phase, suggesting that surgeons were able to identify many high-risk patients without the aid of a prediction tool.
The pragmatic trial design34 employed here had the advantages of flexible delivery, straightforward compliance assessment, an easily measured and clinically important endpoint, and inclusion of all patients, making the study findings applicable to complex and diverse real-world settings35 (Table S2, supporting information). Uniquely, this study included a sustainability phase to assess the long-term effects of the SSI reduction bundle. An important strength was the SSI surveillance system designed to track patients after discharge from hospital. The limitations include an inability to attribute causality to any individual component of the intervention or to distinguish superficial from deep SSI. Although patient compliance was high, some compliance data were self-reported and could not be verified, such as recent and remote history of smoking.
Implementation of the patient care bundle resulted in a sustained cultural change within MSK, as the SSI rate remained low almost 2·5 years after implementation. These findings indicate that SSI rates can be reduced in the long term by hospital-wide implementation of a multidisciplinary care bundle.
Collaborators
Members of the MSK Multidisciplinary SSI Reduction Team are: A. Afonso, A. Aslam, A. Baldwin-Medsker, J. Burns, M. Canny, C. E. Cheavers, N. Cohen, J. Eagan, N. Evans, C. Ferrari, C. Fiordaliso, C. Fitzpatrick, R. Freeman, J. Garcia-Aguilar, M. Gilbert, M. Gonen, J. G. Guillem, M. Hailemariam, J. Hammel, K. Healy, W. Hoskins, L. Isaac, W. Kim, R. Kitzler, K. Levine, A. Marcelli, W. Marx, L. Matthews, C. Monether, G. M. Nash, S. Nolan, H. Ottey, D. Patel, P. Patterson, P. B. Paty, T. Pottinger, A. Prather, M. Riffle, D. Rodrigue, S. Romanoff, T. Russo, P. Samedy, S. K. Seo, K. Sepkowitz, J. J. Smith, D. Sokoli, L. Swift, L. Temple, S. Usiak, C. Vassallo, A. Vincenzino, L. Wall, C. Walters, M. R. Weiser, K. Yeung (Memorial Sloan Kettering Cancer Center, New York, USA).
Supplementary Material
Appendix S1 Supplementary methods
Table S1 Characteristics of the model development cohort (n = 1,471)*
Table S2 The study's scores on the nine domains of PRECIS-2 (Pragmatic Explanatory Continuum Indicator Summary 2)1
Acknowledgements
The authors acknowledge the editorial assistance of A. Gelmis and J. Moore. This study was supported in part by a National Cancer Institute grant (P30 CA008748).
Disclosure: The authors declare no conflict of interest.
Snapshot quiz 18/17

References
- 1. Magill SS, Hellinger W, Cohen J, Kay R, Bailey C, Boland Bet al. Prevalence of healthcare-associated infections in acute care hospitals in Jacksonville, Florida. Infect Control Hosp Epidemiol 2012; 33: 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MAet al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014; 370: 1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scott RD. The Direct Medical Costs of Healthcare Associated Infection in U.S. Hospitals and the Benefits of Prevention; 2009. www.cdc.gov/hai/pdfs/hai/Scott_CostPaper.pdf [accessed 30 January 2018]. [Google Scholar]
- 4. Anderson DJ, Kaye KS. Staphylococcal surgical site infections. Infect Dis Clin North Am 2009; 23: 53–72. [DOI] [PubMed] [Google Scholar]
- 5. Artinyan A, Orcutt ST, Anaya DA, Richardson P, Chen GJ, Berger DH. Infectious postoperative complications decrease long-term survival in patients undergoing curative surgery for colorectal cancer: a study of 12 075 patients. Ann Surg 2015; 261: 497–505. [DOI] [PubMed] [Google Scholar]
- 6. Merkow RP, Ju MH, Chung JW, Hall BL, Cohen ME, Williams MVet al. Underlying reasons associated with hospital readmission following surgery in the United States. JAMA 2015; 313: 483–495. [DOI] [PubMed] [Google Scholar]
- 7. Awad SS. Adherence to Surgical Care Improvement Project measures and post-operative surgical site infections. Surg Infect (Larchmt) 2012; 13: 234–237. [DOI] [PubMed] [Google Scholar]
- 8. Bratzler DW, Houck PM; Surgical Infection Prevention Guideline Writers Workgroup . Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg 2005; 189: 395–404. [DOI] [PubMed] [Google Scholar]
- 9. Surgical Care Improvement Project . Specifications Manual for Joint Commission National Quality Core Measures: Surgical Care Improvement Project; 2010. https://manual.jointcommission.org/releases/archive/TJC2010B/SurgicalCareImprovementProject.html [accessed 30 January 2018]. [Google Scholar]
- 10. Ingraham AM, Cohen ME, Bilimoria KY, Dimick JB, Richards KE, Raval MVet al. Association of Surgical Care Improvement Project infection-related process measure compliance with risk-adjusted outcomes: implications for quality measurement. J Am Coll Surg 2010; 211: 705–714. [DOI] [PubMed] [Google Scholar]
- 11. Larochelle M, Hyman N, Gruppi L, Osler T. Diminishing surgical site infections after colorectal surgery with Surgical Care Improvement Project: is it time to move on? Dis Colon Rectum 2011; 54: 394–400. [DOI] [PubMed] [Google Scholar]
- 12. Stulberg JJ, Delaney CP, Neuhauser DV, Aron DC, Fu P, Koroukian SM. Adherence to Surgical Care Improvement Project measures and the association with postoperative infections. JAMA 2010; 303: 2479–2485. [DOI] [PubMed] [Google Scholar]
- 13. Hawn MT, Vick CC, Richman J, Holman W, Deierhoi RJ, Graham LAet al. Surgical site infection prevention: time to move beyond the Surgical Care Improvement Program. Ann Surg 2011; 254: 494–501. [DOI] [PubMed] [Google Scholar]
- 14. Consensus paper on the surveillance of surgical wound infections. Society for Hospital Epidemiology of America . The Society for Hospital Epidemiology of America; The Association for Practitioners in Infection Control; The Centers for Disease Control; The Surgical Infection Society. Infect Control Hosp Epidemiol 1992; 13: 599–605. [PubMed] [Google Scholar]
- 15. Cima R, Dankbar E, Lovely J, Pendlimari R, Aronhalt K, Nehring Set al. Colorectal surgery surgical site infection reduction program: a National Surgical Quality Improvement Program-driven multidisciplinary single-institution experience. J Am Coll Surg 2013; 216: 23–33. [DOI] [PubMed] [Google Scholar]
- 16. Condon RE, Schulte WJ, Malangoni MA, Anderson-Teschendorf MJ. Effectiveness of a surgical wound surveillance program. Arch Surg 1983; 118: 303–307. [DOI] [PubMed] [Google Scholar]
- 17. Haley RW, Culver DH, White JW, Morgan WM, Emori TG, Munn VPet al. The efficacy of infection surveillance and control programs in preventing nosocomial infections in US hospitals. Am J Epidemiol 1985; 121: 182–205. [DOI] [PubMed] [Google Scholar]
- 18. Keenan JE, Speicher PJ, Thacker JK, Walter M, Kuchibhatla M, Mantyh CR. The preventive surgical site infection bundle in colorectal surgery: an effective approach to surgical site infection reduction and health care cost savings. JAMA Surg 2014; 149: 1045–1052. [DOI] [PubMed] [Google Scholar]
- 19. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol 1999; 20: 250–278. [DOI] [PubMed] [Google Scholar]
- 20. Pronovost PJ, Bo-Linn GW. Preventing patient harms through systems of care. JAMA 2012; 308: 769–770. [DOI] [PubMed] [Google Scholar]
- 21. Wick EC, Hobson DB, Bennett JL, Demski R, Maragakis L, Gearhart SLet al. Implementation of a surgical comprehensive unit-based safety program to reduce surgical site infections. J Am Coll Surg 2012; 215: 193–200. [DOI] [PubMed] [Google Scholar]
- 22. Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove Set al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med 2006; 355: 2725–2732. [DOI] [PubMed] [Google Scholar]
- 23. Berenholtz SM, Pham JC, Thompson DA, Needham DM, Lubomski LH, Hyzy RCet al. Collaborative cohort study of an intervention to reduce ventilator-associated pneumonia in the intensive care unit. Infect Control Hosp Epidemiol 2011; 32: 305–314. [DOI] [PubMed] [Google Scholar]
- 24. Saint S, Greene MT, Krein SL, Rogers MA, Ratz D, Fowler KEet al. A program to prevent catheter-associated urinary tract infection in acute care. N Engl J Med 2016; 374: 2111–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zwarenstein M, Treweek S, Gagnier JJ, Altman DG, Tunis S, Haynes Bet al. ; CONSORT group; Pragmatic Trials in Healthcare (Practihc) group. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ 2008; a2390: 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blackham AU, Farrah JP, McCoy TP, Schmidt BS, Shen P. Prevention of surgical site infections in high-risk patients with laparotomy incisions using negative-pressure therapy. Am J Surg 2013; 205: 647–654. [DOI] [PubMed] [Google Scholar]
- 27. National Nosocomial Infections Surveillance System . National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control 2004; 32: 470–485. [DOI] [PubMed] [Google Scholar]
- 28. Centers for Disease Control and Prevention . Surgical Site Infection (SSI) Event; 2018. www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf [accessed 30 January 2018]. [Google Scholar]
- 29. Biglan A, Ary D, Wagenaar AC. The value of interrupted time-series experiments for community intervention research. Prev Sci 2000; 1: 31–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Waits SA, Fritze D, Banerjee M, Zhang W, Kubus J, Englesbe MJet al. Developing an argument for bundled interventions to reduce surgical site infection in colorectal surgery. Surgery 2014; 155: 602–606. [DOI] [PubMed] [Google Scholar]
- 31. Anthony T, Murray BW, Sum-Ping JT, Lenkovsky F, Vornik VD, Parker BJet al. Evaluating an evidence-based bundle for preventing surgical site infection: a randomized trial. Arch Surg 2011; 146: 263–269. [DOI] [PubMed] [Google Scholar]
- 32. Tanner J, Padley W, Assadian O, Leaper D, Kiernan M, Edmiston C. Do surgical care bundles reduce the risk of surgical site infections in patients undergoing colorectal surgery? A systematic review and cohort meta-analysis of 8515 patients. Surgery 2015; 158: 66–77. [DOI] [PubMed] [Google Scholar]
- 33. Bergquist JR, Thiels CA, Etzioni DA, Habermann EB, Cima RR. Failure of colorectal surgical site infection predictive models applied to an independent dataset: do they add value or just confusion? J Am Coll Surg 2016; 222: 431–438. [DOI] [PubMed] [Google Scholar]
- 34. Ford I, Norrie J. Pragmatic trials. N Engl J Med 2016; 375: 454–463. [DOI] [PubMed] [Google Scholar]
- 35. Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 2015; 350: h2147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supplementary methods
Table S1 Characteristics of the model development cohort (n = 1,471)*
Table S2 The study's scores on the nine domains of PRECIS-2 (Pragmatic Explanatory Continuum Indicator Summary 2)1


