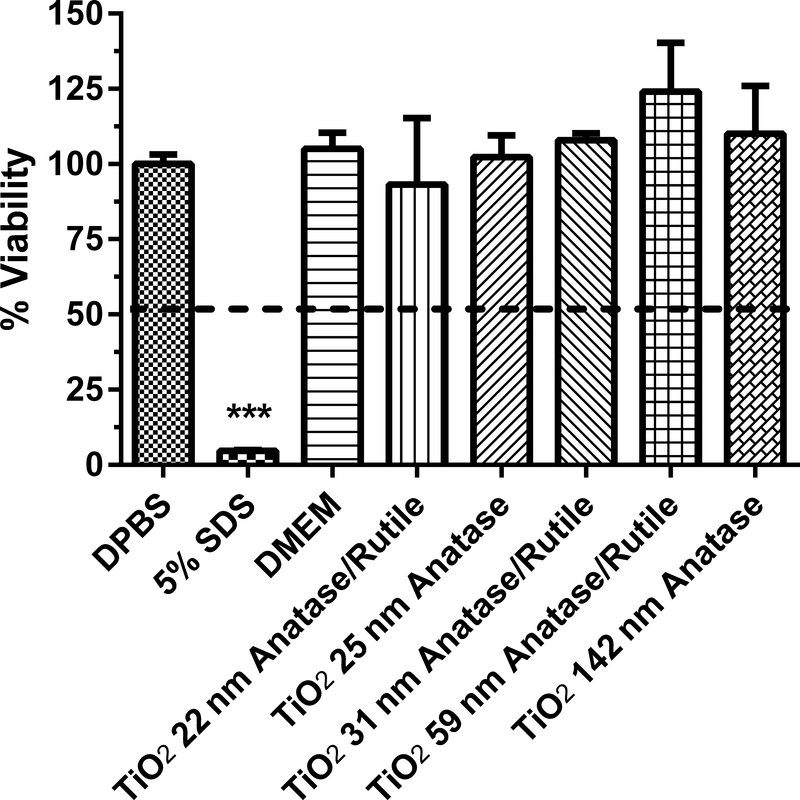
Figure 4.
Human equivalent skin model tissue viability (%) exposed to titanium dioxide nanoparticles of various sizes for 1 h. Data represents mean ± SD, N=3. The concentration of each nanoparticle sample was 1 mg/mL. DPBS (Dulbecco’s phosphate buffered saline) was the negative control. 5% SDS (sodium dodecyl sulfate) was the positive control. DMEM (Dubelco’s minimum essential medium with 10% fetal bovine serum) was the vehicle for the TiO2 nanoparticles. Chemicals with a mean value below the dashed line at 50% viability were considered to be dermal irritants. ***, significantly from negative control, p < 0.001.