Abstract
Type I diabetes mellitus, which affects an estimated 1.5 million Americans, is caused by autoimmune destruction of the pancreatic beta cells that results in the need for life-long insulin therapy. Allogeneic islet transplantation for the treatment of type I diabetes is a therapy in which donor islets are infused intrahepatically, which has led to the transient reversal of diabetes. However, therapeutic limitations of allogeneic transplantation, which include a shortage of donor islets, long-term immunosuppression, and high risk of tissue rejection, have led to the investigation of embryonic or induced pluripotent stem cells as an unlimited source of functional beta-cells. Herein, we investigate the use of microporous scaffolds for their ability to promote the engraftment of stem cell derived pancreatic progenitors and their maturation toward mono-hormonal insulin producing β-cells at a clinically translatable, extrahepatic site. Initial studies demonstrated that microporous scaffolds supported cell engraftment, and their maturation to become insulin positive; however, the number of insulin positive cells and the levels of C-peptide secretion were substantially lower than what was observed with progenitor cell transplantation into the kidney capsule. The scaffolds were subsequently modified to provide a sustained release of exendin-4, which has previously been employed to promote maturation of pancreatic progenitors in vitro and has been employed to promote engraftment of transplanted islets in the peritoneal fat. Transplantation of stem cell derived pancreatic progenitors on scaffolds releasing exendin-4 led to significantly increased C-peptide production compared to scaffolds without exendin-4, with C-peptide and blood glucose levels comparable to the kidney capsule transplantation cohort. Image analysis of insulin and glucagon producing cells indicated that monohormonal insulin producing cells were significantly greater compared to glucagon producing and polyhormonal cells in scaffolds releasing exendin-4, whereas a significantly decreased percentage of insulin-producing cells were present among hormone producing cells in scaffolds without exendin-4. Collectively, a microporous scaffold, capable of localized and sustained delivery of exendin-4, enhanced the maturation and function of pluripotent stem cell derived pancreatic progenitors that were transplanted to a clinically translatable site.
Keywords: Tissue Engineering, Biomaterials, Stem Cells, Cell Transplantation, Type I Diabetes
Graphical Abstract
Introduction
Type I diabetes (T1D), which affects an estimated 1.5 million Americans, is caused by autoimmune destruction of the pancreatic β-cells that currently results in the need for life-long insulin therapy 1–3. Rigorous glucose monitoring combined with insulin administration can manage symptoms in T1D patients; however, secondary microvascular and macrovascular complications eventually afflict most T1D patients 1–3. Cellular therapy holds promise for permanent control of glucose within the physiologically required range, and would allow for endogenous insulin production 4–5. Human allogeneic islet transplantation as a β-cell replacement strategy reached a major milestone in 2000 when investigators achieved diabetes reversal in seven out of seven recipients by hepatic infusion of islets from multiple donor pancreata combined with corticosteroid-free immunosuppression 3. Since then, improvements in islet transplantation have led to 56% of patients transplanted becoming insulin dependent after 3 years 6, with limited long-term function being attributed to immunosuppression protocols and the local milieu within the liver contributes to limited islet engraftment 7–9.
Alternative cell sources for transplantation are being sought as the supply of donor islets is limited 6, 10–14. Pluripotent stem cell derived β-cell therapy is emerging for T1D treatment, since the recent ability to direct stem cell differentiation of renewable pluripotent stem cells toward a β-cell lineage have been developed 15–21. Several milestones have been achieved with the development of culture systems that enable pluripotent stem cells (PSCs) to form definitive endoderm 22, with subsequent development through pancreatic endoderm to endocrine cells capable of synthesizing pancreatic hormones 23. While cells have been differentiated in vitro to various stages of immature β-cells, transplantation in vivo is necessary to complete cell maturation 4, 16, 18, 21, 24–26.
Alternative transplantation sites may be necessary for the transplantation of in vitro derived pancreatic progenitors. Previous reports have primarily employed transplantation of PSC derived pancreatic progenitors into the kidney capsule 15–18, 20–21. Biomaterial scaffold systems have been employed for transplanting the progenitors have, until recently 27, been less efficient relative to transplantation of pancreatic progenitors to the kidney capsule, or have involved components such as Matrigel that are not translational for clinical applications 4, 28–29. A recent report has indicated that encapsulation of immature β-cells within an alginate hydrogel and transplanted to the intraperitoneal space of diabetic mice can restore euglycemia within 14 days following transplantation and provides long-term glucose control 30. Importantly, these cells following transplantation must continue to mature in the presence of signals from the local host microenvironment in vivo.
Herein, we investigated the use of microporous scaffolds for extrahepatic, extrarenal transplantation of pancreatic progenitors, with localized delivery of trophic factors to enhance maturation. We have previously reported on scaffolds for extrahepatic transplantation 31–32 of murine, human, and porcine islets 32–33, which has led to engraftment and long-term function. Furthermore, the scaffolds have been fabricated for the sustained, localized release of exendin-4, a glucagon-like peptide-1 (GLP-1) receptor agonist that stimulates glucose dependent insulin secretion, protects islets against apoptosis, and improves outcome in animal islet transplantation models 34–36. Exendin-4 has also been previously reported to enhance the differentiation of mouse embryonic stem cells to insulin-producing beta cells, primarily by upregulating Neurod1 and Glut2 gene expression 37–38. Taken together, we hypothesize that using PSC-derived pancreatic progenitors along with a cell delivery platform, capable of localized and sustained delivery of trophic factors, will enhance in vivo cell maturation toward monohormonal insulin-producing cells at a clinically translatable site.
Materials and Methods
Microporous PLG Scaffolds
Microporous scaffolds were fabricated as previously described 32–33, 39–43. Briefly, microporous scaffolds were fabricated by compression molding PLG microspheres (75:25 mol ratio d,l-lactide to glycolide) and 250–425 μm salt crystals in a 1:30 ratio of PLG microspheres to salt. The mixture was humidified in an incubator for 7 minutes, and then thoroughly mixed again. Non-layered scaffolds were compression molded with 77.5 mg of polymer-salt mixture; layered scaffolds were compression molded with an inner layer, containing exendin-4 loaded PLG microspheres sandwiched between two 38.75 mg layers 42. Both non-layered and layered scaffolds were compression molded into cylinders 5 mm in diameter by 2 mm in height using a 5mm KBr die (International Crystal Labs, Garfield, NJ) at 1500 psi for 30 seconds. Molded constructs were gas foamed in 800 psi carbon dioxide for 16 hours in a pressure vessel. The vessel was depressurized at a controlled rate for 30 minutes. On day of transplantation, scaffolds were leached in water for 1.5 hours, changing the water once after 1 hour. Scaffolds were sterilized by submersion in 70% ethanol for 30 seconds, and multiple rinses with phosphate buffer solution. Scaffolds were coated with a 1 mg/mL solution of collagen IV for 20 min. prior to cell seeding.
In Vitro Differentiation of PSCs into Pancreatic Progenitor Cells
Pluripotent H1 PSCs (WiCEll, Madison, WI, USA), which are on the list of FDA approved PSC lines and previous reports have demonstrated their capacity for developing into insulin producing cells 16–17, 19, 44–46, were cultured in MTeSR1 media (Stem Cell Technologies, Vancouver, BC, Canada) on tissue culture treated plates (Corning Life Sciences, Tewksbury, MA, USA) over a layer of Geltrex® murine tumor basement membrane extract (Life Technologies/Thermo-Fisher, Waltham, MA, USA). Pluripotent cell clusters were lifted into a single cell suspension using Accutase (Stem Cell Technologies), and plated on Geltrex-coated 6-well tissue culture treated plates (Corning Life Sciences), at a concentration of 1.5*106 cells per well of a 6-well plate. The pluripotent cells were differentiated according to a previously established 15-day differentiation protocol 16, in which fresh growth factors are added fresh daily to direct differentiation through the first four stages of embryonic development: (1) definitive endoderm (3 days), (2) primitive gut tube (3 days), (3) posterior foregut (4 days), and finally (4) pancreatic endoderm and endocrine precursors (5 days). At the end of stage 4, differentiated cells were lifted from the plates using Accutase and seeded on sterilized, collagen IV coated scaffolds at a concentration of 3×106 cells/scaffold.
qRT-PCR
Gene expression of pancreatic markers was assessed to track progress through stages of differentiation. First, cells were pelleted and flash frozen using liquid nitrogen. RNA was isolated (Qiagen; Hilden, Germany) and qRT-PCR was conducted (Qiagen; Hilden, Germany) against key pancreatic differentiation markers, using the primers found on Table 1. Gene expression was calculated based on fold change in comparison to house keeper gene GAPDH, followed by normalization to marker expression in pluripotent ESCs.
Table 1.
Primers of pancreatic differentiation markers used for qRT-PCR
| Gene | Primer | Sequence (5′ to 3′) |
|---|---|---|
| KIR2 | Forward | AAGGAGATGACCAGCCTCAG |
| KIR2 | Reverse | GCCCACGAAAGTTATGAGGA |
| SUR1 | Forward | AAGGAGATGACCAGCCTCAG |
| SUR1 | Reverse | GCCCACGAAAGTTATGAGGA |
| PDX1 | Forward | CCTTTCCCATGGATGAAGTC |
| PDX1 | Reverse | CGTCCGCTTGTTCTCCTC |
| NKX6.1 | Forward | GGGGATGACAGAGAGTCAGG |
| NKX6.1 | Reverse | CGAGTCCTGCTTCTTCTTGG |
| MAFA | Forward | GAGAGCGAGAAGTGCCAACT |
| MAFA | Reverse | TTCTCCTTGTACAGGTCCCG |
| Insulin | Forward | TTCTACACACCCAAGACCCG |
| Insulin | Reverse | CAATGCCACGCTTCTGC |
| Glucagon | Forward | TGCTCTCTCTTCACCTGCTCT |
| Glucagon | Reverse | AGCTGCCTTGTACCAGCATT |
Streptozotocin Induced Diabetic Mouse Model
Male NSG mice (Jackson Laboratories, Bar Harbor, ME, USA) between 8 and 12 weeks of age received an intraperitoneal injection of 130 mg/kg streptozotocin 4–7 days prior to transplant. (Sigma Aldrich, St Louis, MO). Each day leading up to surgery, blood glucose levels were measured using the tail vein prick technique (Accu-chek/Roche, Basel, Switzerland) and body weight was recorded. The criteria to include a mouse in the study was the following: glucose readings above 350 mg/dL on two consecutive days leading up to transplant surgeries, and no more than a 20% reduction in weight since streptozotocin injection. A sustained release insulin pellet (LinBit, LinShin Canada, Inc., Scarborough, ON, Canada), containing USP grade bovine insulin, was inserted subcutaneously at time of surgery to assist in mouse health during engraftment of transplanted cells, and every 4 weeks until the end point of the study. Following surgery, glucose and body weight for each mouse were measured three times per week. All studies involving mice were approved by the University of Michigan Animal Care and Use Committee.
Transplantation of pancreatic progenitors to streptozotocin-induced diabetic NSG mice
The day before scaffold seeding, 100 mg of fibrinogen (EMD Millipore, Billerica, MA, USA) was dissolved in 2 ml of tris-buffered saline (TBS) at 37 °C for 2 hours. The solution was added to a 10,000 MW cut-off dialyzing cassette and dialyzed overnight in 1 liter of TBS. The dialyzed fibrinogen solution was estimated to be approximately 20 mg/ml, and this solution was diluted to 4mg/ml in TBS. On the day of surgery, stage 4 pancreatic progenitors were lifted from tissue culture plates using Accutase, and 3×106 cells were resuspended in 6 μl thrombin mix (50 U/ml thrombin, 50 mM CaCl2, in TBS). 6 ul of 4 mg/ml fibrin solution was added to the mix, and the cell suspension was promptly added to semi-dried scaffolds. Mice were anesthetized with 2% isoflurane until loss of consciousness was confirmed by pinch reflex test. The abdominal area was shaved and sterilized with betadine and ethanol. An approximate 5 mm incision was made in the peritoneal wall, in the middle of the abdominal region, and the epididymal fat pads (EFP) were located and unwrapped outside of the abdominal wall. Scaffolds were wrapped within the epididymal fat pad, then placed back into the peritoneal cavity 47. Kidney capsule transplantation was adapted from a previously published report 16. 5 million cells were pelleted and suspended in a fibrin thrombin mix. The mix was injected into the kidney capsule. Mice were given a dose of 0.005 mg/gram body weight Rimadyl carprofen (Zoetis, Florham Park, NJ, USA) and allowed free access to food and water post operatively.
Serum Collection and Human C-peptide Analysis
Once every 4 weeks, blood serum was collected to quantify circulating human C-peptide levels. Mice were fasted 5 hours before blood collection. 30 minutes prior to blood collection, mice received an intraperitoneal injection of 2 g/kg glucose in the form of 20% glucose. Approximately 200 μl of blood was collected from the saphenous vein during the period between 30–60 minutes post glucose stimulation. Serum was collected from whole blood by 20 minutes of coagulation followed by centrifuging at 2000 g for 20 minutes. Isolated serum was analyzed for human C-peptide levels using the manufacturer’s provided ELISA protocol (AbCam, Cambridge, MA, USA).
Immunostaining
Immunostaining of in vitro cell differentiation was performed at stages 0 (pluripotent), 1 (definitive endoderm), 2 (posterior foregut), and 4 (pancreatic progenitors). Cells differentiated in 6-well plates were fixed using 4% paraformaldehyde (Electron Microscopy Sciences; Hatfield, PA, U.S.A.), permeabilized using 0.5% Triton in tris-buffered saline, blocked using normal donkey serum, and stained for key differentiation markers at each stage (Oct3/4 and Sox17 for stages 0 and 1, FoxA2 and HNF4-alpha for stage 2; C-peptide, Nkx6.1, insulin and glucagon for stage 4) and cell nuclei were counterstained with DAPI. For immunohistochemical assessment of in vivo cell maturation, scaffolds were removed at 4 and 8 weeks post-transplant. Epididymal fat pads were isolated and a majority of the fat sounding the scaffold was removed with forceps. Scaffolds were cryopreserved in isopentane cooled on dry ice, then embedded within O.C.T. embedding medium, and cryosectioned. Sections were stained for C-peptide, human mitochondria (HuMit), insulin, glucagon, and cell nuclei were counterstained with DAPI. Digital images were acquired with a MicroFire digital camera (Optronics, Goleta, CA) connected to an Olympus BX-41 fluorescence microscope (Olympus, Center Valley, PA, USA). Image quantification was conducted using MATLAB software using an object-based colocalization analysis. DAPI+ cells were identified and quantified by applying Otsu’s thresholding method, the watershed transform, and individual cluster thresholding. Then, each cell’s colocalization with immunofluorescent markers was quantified.
Statistics
All statistical analysis was conducted using Prism graphing and data analysis software (GraphPad Software, Inc., La Jolla, CA, USA). Values were reported as the mean ± SEM.
Results
In vitro differentiation of pancreatic progenitors and seeding on scaffolds
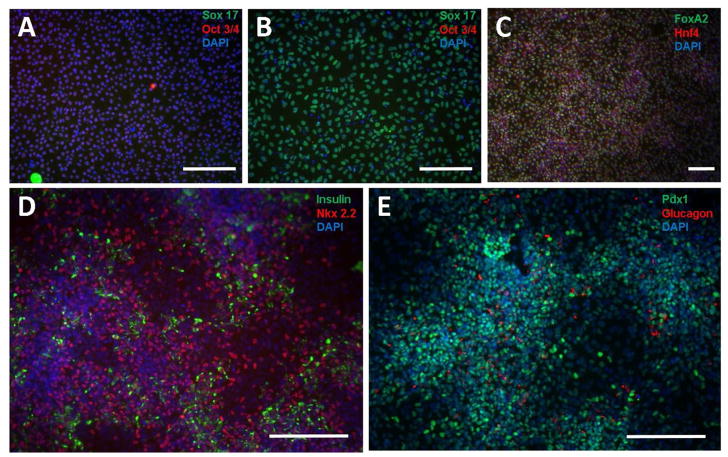
PSCs were cultured and differentiated in vitro to pancreatic progenitors following a previously established protocol by Rezania, et. al., 16–17. Pluripotent cells were stained and imaged for Sox17 (green) and Oct3/4 (red), 24 h. after plating, prior to the induction of differentiation to the definitive endoderm lineage, indicating that a population of Oct3/4 expressing, undifferentiated pluripotent cells was obtained (Figure 1A). Three days after induction of differentiation to the definitive endoderm lineage, staining for Oct3/4 and Sox17 indicates the presence of a Sox17-expressing cell population (Fig. 1B), demonstrating the differentiation of pluripotent stem cells to definitive endoderm cells. Furthermore, upon differentiating cells to the primitive gut tube lineage (stage 2), cellular staining for FoxA2 and Hnf4, was prevalent. Successful differentiation into pancreatic progenitors was verified by immunostaining for insulin and glucagon, as well as Pdx1- and Nkx2.2-expression (Fig 1D, E). Additionally, qRT-PCR was used to quantify the expression of key factors involved in the differentiation from PSCs to pancreatic progenitors throughout in vitro differentiation (Fig. 2). The qRT-PCR results indicate the progressive increase in expression of Ngn3 and NeuroD, which are markers for beta-cell maturity, as well as co-expression of Pdx1 and Nkx6.1, which are markers of beta cell fate. Furthermore, expression of Sur1, an ATP channel protein, and Kir2, a calcium channel-closer, increased at stages 3 and 4, corresponding to the expression of insulin and glucagon.
Figure 1. In vitro differentiation of PSCs to pancreatic progenitor cells.
H1 pluripotent PSCs were seeded to Matrigel-coated 6-well plates at a density of 1.5 million cells per well and pluripotent cells were imaged for sox17 (green) and oct3/4 (red) prior to beginning the differentiation (A), definitive endoderm cells were imaged for sox17 (green) and oct3/4 (red) following stage 1 of the differentiation (B), primitive gut tube cells were imaged for FoxA2 (green) and Hnf4 (red) (C), and pancreatic progenitor cells were stained for insulin (green) and Nkx2.2 (red) (D) as well as Pdx1 (green) and glucagon (red) (E) following the fourth stage of differentiation. All cells were counterstained with DAPI (blue). Scale bars for all images = 200 μm.
Figure 2.
qRT-PCR of in vitro differentiation of human PSC derived pancreatic progenitors.
Transplantation of PSC-derived pancreatic progenitors to epididymal fat pad
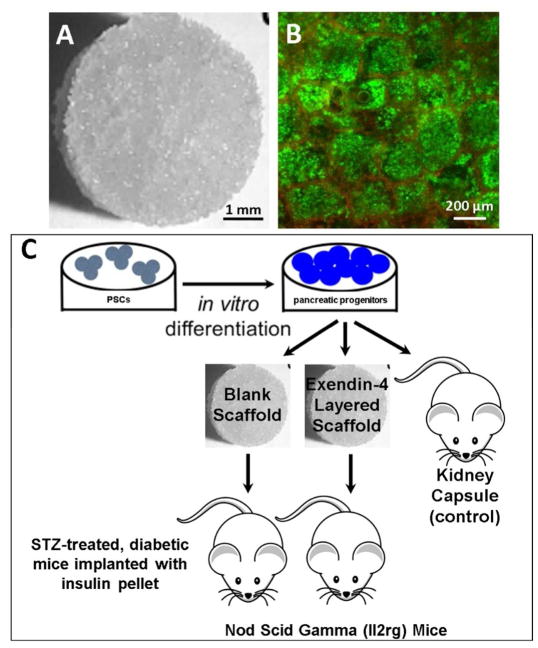
Following in vitro differentiation, the pancreatic progenitors were seeded onto collagen IV-coated microporous PLG scaffolds (Fig. 3A) and pancreatic progenitor live/dead staining at 1 hr after seeding indicated good cell viability (Fig. 3B) and a homogenous distribution of cells throughout the pores of the PLG scaffold (Fig. 3B).
Figure 3.
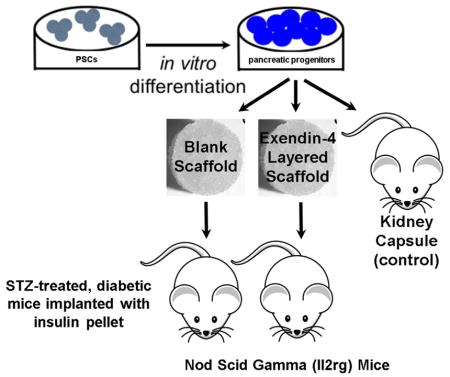
Microporous PLG scaffold morphology (A) and live/dead stain of seeded pancreatic progenitors (B). Schematic of experimental workflow (C). PSCs were differentiated in vitro to pancreatic progenitors, then seeded to either microporous PLG scaffolds without exendin-4 or to scaffolds containing an encapsulated exendin-4 microparticles within an inner layer disk. Scaffolds were subsequently transplanted to the epididymal fat pads of STZ-treated, diabetic NSG mice.
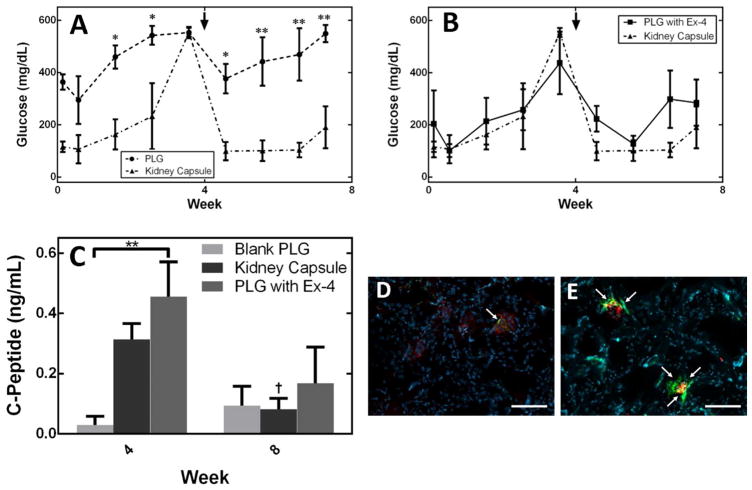
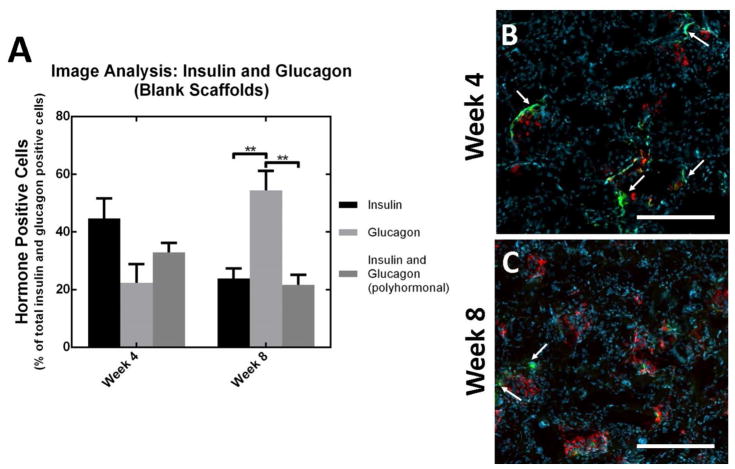
Microporous PLG scaffolds seeded with pancreatic progenitors were transplanted into the epididymal fat pads of NSG mice (Figure 3C) 48, with transplantation under the kidney capsule serving as a control. The function of these transplanted cells was assessed through blood glucose and human C-peptide measurements in serum (Figure 4A, B, C). Blood glucose levels for the kidney capsule transplant cohort stabilized (between approx. 100–250 mg/dL) upon administration of insulin pellets at week 4. In contrast, mice receiving pancreatic progenitors seeded to PLG scaffolds had high glucose levels (approx. 400–600 mg/dL) that were statistically different from mice transplanted with pancreatic progenitors under the kidney capsule, despite the presence of insulin pellets in both cohorts (Figure 4A). The concentration of human C-peptide in serum, which was 10.6–fold greater (n.s. alpha=0.05) for cells transplanted into the kidney capsule compared to cells transplanted on the PLG scaffolds into the peritoneal fat at 4 weeks. At week 8, C-peptide production for cells within the kidney capsule decreased and is not significantly different (n.s. alpha=0.05) relative to the cells transplanted on PLG within the epidiymal fat pads. Cellular co-localization of both human mitochondria and C-peptide at week 4 (Figure 4D), indicates that human cells are present within the scaffold, but producing very small amounts of C-peptide, which reflects the measurement of C-peptide at week 4 (Figure 4C). Image analysis of insulin and glucagon producing cells indicates no significant differences between insulin, glucagon, and polyhormonal cells (insulin and glucagon producing cells) at week 4 and glucagon producing cells were significantly greater (p<0.01) than insulin producing and polyhormonal cells at week 8 (Figure 5).
Figure 4.
Blood glucose measurements of mice transplanted with pancreatic progenitor cells, seeded to blank PLG scaffolds or transplantation of cells under the kidney capsule (A, arrow indicates administration of insulin pellet). Blood glucose measurements of mice transplanted with pancreatic progenitors seeded to PLG scaffolds containing exendin-4 or transplantation of cells under the kidney capsule (B, arrow indicates administration of insulin pellet). Human C-peptide in serum for a cohort of mice receiving macroporous nonlayered PLG scaffolds containing PSC-derived pancreatic progenitors (n=4), a cohort of mice receiving layered PLG scaffolds containing exendin-4 (n-4), and a cohort of mice transplanted with PSC-derived pancreatic progenitors under the kidney capsule (n=4) (C). Histology of a blank scaffold (D) and exendin-4 layered scaffold (E) removed at week 4, stained for c-peptide (green), human mitochondria (red), and counterstained with DAPI (blue). All values reported as the average +/− standard error; * indicates p<0.05, ** indicates p<0.01, † indicates p<0.05 difference between week 4 and week 8 among a cohort of mice. Arrows on images (D and E) indicate regions of C-peptide/human mitochondria co-expression. All scale bars for images D&E = 100 μm.
Figure 5.
Image quantification of monohormonal insulin producing cells and polyhormonal insulin/glucagon producing cells for blank scaffolds at weeks 4 and 8 (A). Histology of blank layered scaffolds (B,C) removed at weeks 4 and 8, stained for insulin (green), glucagon (red), and counterstained with DAPI (blue). All values reported as the average +/− standard error; ** indicates p<0.01. Arrows indicate regions of monohormonal insulin production. Scale bars = 200 μm
Exendin-4 delivery from scaffold enhances pancreatic progenitor function
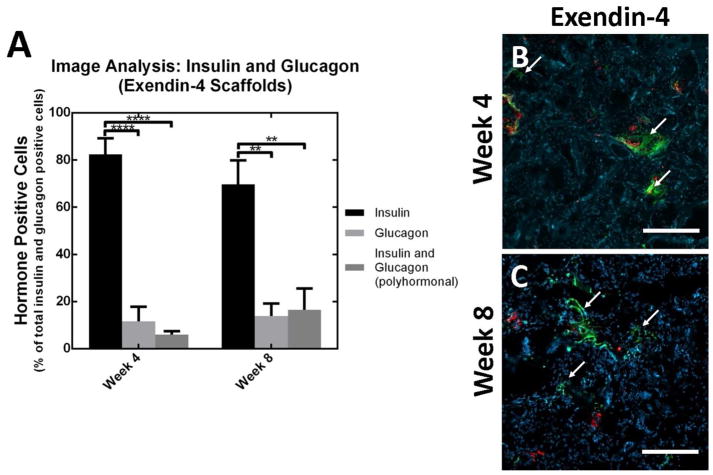
We subsequently investigated the localized delivery of trophic factors from scaffolds as a means to enhance maturation and function of the transplanted pancreatic progenitors. Blood glucose levels of mice transplanted with scaffolds containing exendin-4 were statistically similar to blood glucose levels of mice transplanted with pancreatic progenitors under the kidney capsule (n.s. alpha=0.05, Figure 4B). Average measurements of serum C-peptide for cells transplanted on exendin-4 containing scaffolds to the fat pad were 15.7-fold greater relative to cells transplanted on blank PLG scaffolds to the fat pad (p<0.01) and 1.5-fold greater relative to cell transplantation to the kidney capsule (n.s. alpha=0.05) (Figure 4C). Cellular co-localization of both human mitochondria and C-peptide at week 4 (Figure 4E), indicates that human cells are present within the scaffold and producing C-peptide. Furthermore, image analysis indicates that the presence of insulin producing cells was significantly greater than glucagon producing and polyhormonal cells at weeks 4 (p<0.0001) and 8 (p<0.01) (Figure 6).
Figure 6.
Image quantification of monohormonal insulin producing cells and polyhormonal insulin/glucagon producing cells for exendin-4 layered scaffolds at weeks 4 and 8 (A). Histology of exendin-4 layered scaffolds (B,C) removed at weeks 4 and 8, stained for insulin (green), glucagon (red), and counterstained with DAPI (blue). All values reported as the average +/− standard error; ** indicates p<0.01; **** indicates p<0.0001. Arrows indicate regions of monohormonal insulin production. Scale bars = 200 μm.
Discussion
Allogeneic islet transplantation for the treatment of T1D is a therapy in which donor islets are infused intrahepatically, which has led to the transient reversal of diabetes. However, allogenic transplantation has several therapeutic limitations, which include a shortage of donor islets, long-term immunosuppression, and high risk of tissue rejection. All of these limitations have led to the investigation of embryonic or induced pluripotent stem cells as an unlimited source of functional β-cells. Successful differentiation into pancreatic progenitors was verified by immunostaining for insulin- and glucagon-producing cells as well as Pdx1- and Nkx2.2-expressing cells (Fig 1D, E) and indicate the differentiation of PSCs into pancreatic progenitors that have the potential to mature into β-cells, as shown by the presence of both Pdx1 and Nkx2.2, as well as the presence of insulin-producing cells. The qRT-PCR results suggest differentiation into pancreatic progenitors, as evidenced by significant upregulation in expression of markers for pancreatic tissue between stages 0 and 4 of differentiation and analogous to values in a previously reported study by Rezania, et.al., 2012 16. More recently, protocols have been developed for the differentiation of PSCs to stage 6 or 7 immature β-cells in vitro 18, 21, 26, 30. For both pancreatic progenitors or later stage immature β-cells, further maturation is necessary following transplantation 26, with instructive cues being provided by the in vivo environment. Biomaterial platforms provide the opportunity to facilitate the organization of cells into structures, or provide cues, that can enhance the in vivo maturation and ultimately function.
Microporous scaffolds were employed for transplantation of the cells at a clinically translatable site, which provides for integration with the host tissue similar to kidney capsule transplants, yet distinct from the isolation of cells from the host obtained with encapsulation systems. Transplantation of progenitor cells into the kidney capsule has been the primary method by which these cells are able to survive, mature, and ultimately normalize blood glucose levels 16, 18, 21. For translational purposes, studies have been investigating extra-renal sites, as well as extrahepatic sites in order to avoid the instant blood mediated inflammatory reaction 49 and may enable retrieval of the cells should it become necessary. Herein, we employed the epididymal fat pad, which has similar properties as the omentum that has emerged as a leading candidate for clinical trials. The peritoneal fat is a relatively large tissue that could accommodate a volume of transplanted cells or, more likely, devices such as cell pouches and scaffolds that contain the transplanted cells 49–50. The integration of the host tissue with the transplanted cells offers the potential to vascularize the cells, which provides the opportunity to sense blood glucose and distribute insulin. However, our results indicated that the cells did not produce C-peptide at levels comparable to the kidney capsule, which suggests that the host tissue interactions in the peritoneal fat did not readily support the maturation and function of the transplanted cells. Additionally, blood glucose levels measured between the two cohorts of mice suggested that transplantation of pancreatic progenitors into epididymal fat site was less effective at supporting β-cell function relative to transplantation under the kidney capsule.
Modulation of the local environment has the potential to augment the endogenous properties of the transplant microenvironment to enhance differentiation of maturing pancreatic progenitors towards the mature β-cell fate 4, 28–29. In a previous investigation using microporous PLG scaffolds for human islet transplantation to diabetic murine models 31–33, 40–42, the trophic factor exendin-4 was delivered as a means to enhance the glucose stimulated insulin secretion from the transplanted islets. The localized release of exendin-4 was applied herein to influence pancreatic progenitor survival and differentiation following transplantation. exendin-4 has also been previously reported to enhance the differentiation of mouse embryonic stem cells to insulin-producing β-cells, primarily by upregulating Neurod1 and Glut2 gene expression 37–38.
We report that modifying this post-transplant microenvironment by sustained, localized delivery of exendin-4 was found to significantly enhance the survival and differentiation of the pancreatic progenitors when transplanted to a clinically translatable site – the peritoneal fat (epididymal fat pads). The studies herein employed scaffolds coated with collagen IV as a means to support and enhance the survival and maturation of pluripotent cells since previous reports have demonstrated that ECM proteins, such as collagen IV and laminin, are key components of the pancreatic basement membrane that are necessary for maintaining islet morphology as well as enhancing β-cell proliferation and insulin gene expression 51–52. This investigation demonstrated that exendin-4 release to transplanted pancreatic progenitors significantly increased C-peptide production relative to blank control scaffolds (0.6 ± 0.1 ng/mL C-peptide in mice with exendin-4 and 0.3 ± 0.1 ng/mL in mice containing blank control scaffolds; p<0.05). Also, the percentage of insulin producing cells in scaffolds releasing exendin-4 was 4–5 fold greater than glucagon producing and polyhormonal cells and significantly greater than the percentage of insulin producing cells in scaffolds without exendin-4, where insulin producing cell percentage was not significantly greater than glucagon producing and polyhormonal cells. These results support the potential of exendin-4 to enhance the in vivo maturation of the progenitor cells toward mature β-cells.
Conclusions
These studies investigated microporous PLG scaffolds and their ability to modulate the microenvironment to promote the in vivo maturation of human PSC derived pancreatic progenitors, with a long-term goal to develop scaffolds that support engraftment and function of immature β-cells at clinically translatable, extrahepatic sites. Transplantation of the progenitor cells into the peritoneal fat did not support maturation and function at levels observed in the kidney capsule. We hypothesized that sustained release of a trophic factor into the microenvironment that has been reported to enhance maturation would enhance the function of the transplanted cells in the peritoneal fat. The localized, sustained delivery of exendin-4 promoted the in vivo development of PSC derived pancreatic progenitors into mature insulin producing beta-cells, with levels of C-peptide comparable to cell transplanted under the kidney capsule, improved glycemic control compared to cells transplanted on scaffolds without exendin-4, and significantly greater percentages of insulin producing cells compared to glucagon and polyhormonal cells 4 and 8 weeks following transplantation. These studies demonstrate that modification of the microenvironment with a locally delivered trophic factor can enhance the function of transplanted progenitor cells, which may be a component of the system for the transplantation of progenitor cells that will ultimately restore euglycemia in patients with Type 1 diabetes.
Acknowledgments
Funding: These studies were supported in part by NIH Grants EB-024410 (L.D.S.) and JDRF.
References
- 1.Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes. 2008;57(12):3169–3176. doi: 10.2337/db08-1084. 57/12/3169 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes. 2006;55(5):1463–1469. doi: 10.2337/db05-1423. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 4.Kroon E, Martinson L, Kadoya K, Bang A, Kelly O, Eliazer S, Young H, Richardson M, Smart N, Cunningham J, Agulnick A, D’Amour K, Carpenter M, Baetge E. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 5.Zhang D, Jiang W, Liu M, Sui X, Yin X, Chen S, Shi Y, Deng H. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009;19(4):429–438. doi: 10.1038/cr.2009.28. [DOI] [PubMed] [Google Scholar]
- 6.Barton F, Rickels M, Alejandro R, Hering B, Wease S, Naziruddin B, Oberholzer J, Odorico J, Garfinkel M, Levy M, Pattou F, Berney T, Secchi A, Messinger S, Senior P, Maffi P, Posselt A, Stock P, Kaufman D, Luo X, Kandeel F, Cagliero E, Turgeon N, Witkowski P, Naji A, O’Connell P, Greenbaum C, Kudva Y, Brayman K, Aull M, Larsen C, Kay T, Fernandez L, Vantyghem M, Bellin M, Shapiro A. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care. 2012;35(7):1436–1445. doi: 10.2337/dc12-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kenyon NS, Chatzipetrou M, Masetti M, Ranuncoli A, Oliveira M, Wagner JL, Kirk AD, Harlan DM, Burkly LC, Ricordi C. Long-term survival and function of intrahepatic islet allografts in rhesus monkeys treated with humanized anti-CD154. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(14):8132–8137. doi: 10.1073/pnas.96.14.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robertson RP. Islet Transplantation a Decade Later and Strategies for Filling a Half-Full Glass. Diabetes. 2010;59(6):1285–1291. doi: 10.2337/db09-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srinivasan P, Huang GC, Amiel SA, Heaton ND. Islet cell transplantation. Postgraduate Medical Journal. 2007;83(978):224–229. doi: 10.1136/pgmj.2006.053447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emamaullee J, Shapiro A. Factors influencing the loss of beta-cell mass in islet transplantation. Cell Transplant. 2007;16(1):1–8. [PubMed] [Google Scholar]
- 11.Alejandro R, Barton F, Hering B, Wease S. 2008 Update from the Collaborative Islet Transplant Registry. Transplantation. 2008;86(12):1783–1788. doi: 10.1097/TP.0b013e3181913f6a. [DOI] [PubMed] [Google Scholar]
- 12.Lakey J, Burridge P, Shapiro A. Technical aspects of islet preparation and transplantation. Transpl Int. 2003;16(9):613–632. doi: 10.1007/s00147-003-0651-x. [DOI] [PubMed] [Google Scholar]
- 13.Lakey J, Mirbolooki M, Shapiro A. Current status of clinical islet cell transplantation. Methods Mol Biol. 2006;333:47–104. doi: 10.1385/1-59745-049-9:47. [DOI] [PubMed] [Google Scholar]
- 14.Balamurugan A, Bottino R, Giannoukakis N, Smetanka C. Prospective and challenges of islet transplantation for the therapy of autoimmune diabetes. Pancreas. 2006;32(3):231–243. doi: 10.1097/01.mpa.0000203961.16630.2f. [DOI] [PubMed] [Google Scholar]
- 15.Bruin J, Rezania A, Xu J, Narayan K, Fox J, O’Neil J, Kieffer T. Maturation and function of human embryonic stem cell-derived pancreatic progenitors in macroencapsulation devices following transplant into mice. Diabetologia. 2013 doi: 10.1007/s00125-013-2955-4. [DOI] [PubMed] [Google Scholar]
- 16.Rezania A, Bruin J, Riedel M, Mojibian M, Asadi A, Xu J, Gauvin R, Narayan K, Karanu F, O’Neil J, Ao Z, Warnock G, Kieffer T. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61(8):2016–2029. doi: 10.2337/db11-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rezania A, Bruin J, Xu J, Narayan K, Fox J, O’Neil J, Kieffer T. Enrichment of human embryonic stem cell-derived NKX6.1-expressing pancreatic progenitor cells accelerates the maturation of insulin-secreting cells in vivo. Stem Cells. 2013 doi: 10.1002/stem.1489. [DOI] [PubMed] [Google Scholar]
- 18.Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, O’Dwyer S, Quiskamp N, Mojibian M, Albrecht T, Yang YHC, Johnson JD, Kieffer TJ. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nature Biotechnology. 2014;32(11):1121–1133. doi: 10.1038/nbt.3033. [DOI] [PubMed] [Google Scholar]
- 19.Chen S, Borowiak M, Fox J, Maehr R, Osafune K, Davidow L, Lam K, Peng L, Schreiber S, Rubin L, Melton D. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol. 2009;5(4):258–265. doi: 10.1038/nchembio.154. [DOI] [PubMed] [Google Scholar]
- 20.Pagliuca FW, Melton DA. How to make a functional beta-cell. Development. 2013;140(12):2472–2483. doi: 10.1242/dev.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pagliuca FW, Millman JR, Gurtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA. Generation of Functional Human Pancreatic beta Cells In Vitro. Cell. 2014;159(2):428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23(12):1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 23.D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24(11):1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 24.Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D’Amour KA, Carpenter MK, Baetge EE. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 25.Russ HA, Parent AV, Ringler JJ, Hennings TG, Nair GG, Shveygert M, Guo TX, Puri S, Haataja L, Cirulli V, Blelloch R, Szot GL, Arvan P, Hebrok M. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. Embo Journal. 2015;34(13):1759–1772. doi: 10.15252/embj.201591058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agulnick AD, Ambruzs DM, Moorman MA, Bhoumik A, Cesario RM, Payne JK, Kelly JR, Haakmeester C, Srijemac R, Wilson AZ, Kerr J, Frazier MA, Kroon EJ, D’Amour KA. Insulin-Producing Endocrine Cells Differentiated In Vitro From Human Embryonic Stem Cells Function in Macroencapsulation Devices In Vivo. Stem Cells Translational Medicine. 2015;4(10):1214–1222. doi: 10.5966/sctm.2015-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weizman A, Michael I, Wiesel-Motiuk N, Rezania A, Levenberg S. The effect of endothelial cells on hESC-derived pancreatic progenitors in a 3D environment. Biomaterials Science. 2014;2(11):1706–1714. doi: 10.1039/c4bm00304g. [DOI] [PubMed] [Google Scholar]
- 28.Kelly O, Chan M, Martinson L, Kadoya K, Ostertag T, Ross K, Richardson M, Carpenter M, D’Amour K, Kroon E, Moorman M, Baetge E, Bang A. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat Biotechnol. 2011;29(8):750–756. doi: 10.1038/nbt.1931. [DOI] [PubMed] [Google Scholar]
- 29.Schulz T, Young H, Agulnick A, Babin M, Baetge E, Bang A, Bhoumik A, Cepa I, Cesario R, Haakmeester C, Kadoya K, Kelly J, Kerr J, Martinson L, McLean A, Moorman M, Payne J, Richardson M, Ross K, Sherrer E, Song X, Wilson A, Brandon E, Green C, Kroon E, Kelly O, D’Amour K, Robins A. A scalable system for production of functional pancreatic progenitors from human embryonic stem cells. PLoS One. 2012;7(5):e37004. doi: 10.1371/journal.pone.0037004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vegas AJ, Veiseh O, Gurtler M, Millman JR, Pagliuca FW, Bader AR, Doloff JC, Li J, Chen M, Olejnik K, Tam HH, Jhunjhunwala S, Langan E, Aresta-Dasilva S, Gandham S, McGarrigle JJ, Bochenek MA, Hollister-Lock J, Oberholzer J, Greiner DL, Weir GC, Melton DA, Langer R, Anderson DG. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nature Medicine. 2016;22(3):306–311. doi: 10.1038/nm.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blomeier H, Zhang X, Rives C, Brissova M, Hughes E, Baker M, Powers AC, Kaufman DB, Shea LD, Lowe WLJ. Polymer Scaffolds as Synthetic Microenvironments for Extrahepatic Islet Transplantation. Transplantation. 2006;82(4):452–459. doi: 10.1097/01.tp.0000231708.19937.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salvay D, Rives C, Zhang X, Chen F, Kaufman D, Lowe WJ, Shea L. Extracellular matrix protein-coated scaffolds promote the reversal of diabetes after extrahepatic islet transplantation. Transplantation. 2008;85(10):1456–1464. doi: 10.1097/TP.0b013e31816fc0ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yap W, Salvay D, Silliman M, Zhang X, Bannon Z, Kaufman D, Lowe WJ, Shea L. Collagen IV-Modified Scaffolds Improve Islet Survival and Function and Reduce Time to Euglycemia. Tissue Eng Part A. 2013 doi: 10.1089/ten.TEA.2013.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duncanson S, Sambanis A. Dual factor delivery of CXCL12 and Exendin-4 for improved survival and function of encapsulated beta cells under hypoxic conditions. Biotechnology and Bioengineering. 2013;110(8):2292–2300. doi: 10.1002/bit.24872. [DOI] [PubMed] [Google Scholar]
- 35.Sharma A, Sorenby A, Wernerson A, Efendic S, Kumagai-Braesch M, Tibell A. Exendin-4 treatment improves metabolic control after rat islet transplantation to athymic mice with streptozotocin-induced diabetes. Diabetologia. 2006;49(6):1247–1253. doi: 10.1007/s00125-006-0251-2. [DOI] [PubMed] [Google Scholar]
- 36.Toyoda K, Okitsu T, Yamane S, Uonaga T, Liu XB, Harada N, Uemoto S, Seino Y, Inagaki N. GLP-1 receptor signaling protects pancreatic beta cells in intraportal islet transplant by inhibiting apoptosis. Biochemical and Biophysical Research Communications. 2008;367(4):793–798. doi: 10.1016/j.bbrc.2008.01.046. [DOI] [PubMed] [Google Scholar]
- 37.Zhao QS, Yang YZ, Hu J, Shan ZY, Wu YS, Lei L. Exendin-4 enhances expression of Neurodl and Glut2 in insulin-producing cells derived from mouse embryonic stem cells. Archives of Medical Science. 2016;12(1):199–207. doi: 10.5114/aoms.2016.57596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong RSY. Extrinsic Factors Involved in the Differentiation of Stem Cells into Insulin-Producing Cells: An Overview. Experimental Diabetes Research. 2011 doi: 10.1155/2011/406182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blomeier H, Zhang X, Rives C, Brissova M, Hughes E, Baker M, Powers AC, Kaufman DB, Shea LD, Lowe WL., Jr Polymer scaffolds as synthetic microenvironments for extrahepatic islet transplantation. Transplantation. 2006;82(4):452–459. doi: 10.1097/01.tp.0000231708.19937.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gibly RF, Zhang X, Lowe WL, Jr, Shea LD. Porous Scaffolds Support Extrahepatic Human Islet Transplantation Engraftment and Function in Mice. Cell Transplantation. 2013;22(5):811–819. doi: 10.3727/096368912x636966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gibly RF, Zhang XM, Graham ML, Hering BJ, Kaufman DB, Lowe WL, Shea LD. Extrahepatic islet transplantation with microporous polymer scaffolds in syngeneic mouse and allogeneic porcine models. Biomaterials. 2011;32(36):9677–9684. doi: 10.1016/j.biomaterials.2011.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hlavaty KA, Gibly RF, Zhang X, Rives CB, Graham JG, Lowe WL, Jr, Luo X, Shea LD. Enhancing Human Islet Transplantation by Localized Release of Trophic Factors From PLG Scaffolds. American Journal of Transplantation. 2014;14(7):1523–1532. doi: 10.1111/ajt.12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kheradmand T, Wang S, Gibly RF, Zhang X, Holland S, Tasch J, Graham JG, Kaufman DB, Miller SD, Shea LD, Luo X. Permanent protection of PLG scaffold transplanted allogeneic islet grafts in diabetic mice treated with ECDI-fixed donor splenocyte infusions. Biomaterials. 2011;32(20):4517–4524. doi: 10.1016/j.biomaterials.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borowiak M, Maehr R, Chen S, Chen A, Tang W, Fox J, Schreiber S, Melton D. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell. 2009;4(4):348–358. doi: 10.1016/j.stem.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lammert E, Cleaver O, Melton D. Role of endothelial cells in early pancreas and liver development. Mech Dev. 2003;120(1):59–64. doi: 10.1016/s0925-4773(02)00332-5. [DOI] [PubMed] [Google Scholar]
- 46.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294(5542):564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- 47.Wang K, Wang X, Han CS, Chen LY, Luo Y. Scaffold-supported Transplantation of Islets in the Epididymal Fat Pad of Diabetic Mice. Jove-Journal of Visualized Experiments. 2017;(125) doi: 10.3791/54995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weaver JD, Headen DM, Aquart J, Johnson CT, Shea LD, Shirwan H, Garcia AJ. Vasculogenic hydrogel enhances islet survival, engraftment, and function in leading extrahepatic sites. Science Advances. 2017;3(6) doi: 10.1126/sciadv.1700184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Merani S, Toso C, Emamaullee J, Shapiro AMJ. Optimal implantation site for pancreatic islet transplantation. British Journal of Surgery. 2008;95(12):1449–1461. doi: 10.1002/bjs.6391. [DOI] [PubMed] [Google Scholar]
- 50.Pellicciaro M, VI, Lanzoni G, Tisone G, Ricordi C. The greater omentum as a site for pancreatic islet transplantation. CellR4. 2017;5(3):e2410. [PMC free article] [PubMed] [Google Scholar]
- 51.Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fassler R, Gu GQ, Gerber HP, Ferrara N, Melton DA, Lammert E. The vascular basement membrane: A niche for insulin gene expression and beta cell proliferation. Developmental Cell. 2006;10(3):397–405. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 52.Stendahl JC, Kaufman DB, Stupp SI. Extracellular Matrix in Pancreatic Islets: Relevance to Scaffold Design and Transplantation. Cell Transplantation. 2009;18(1):1–12. doi: 10.3727/096368909788237195. [DOI] [PMC free article] [PubMed] [Google Scholar]