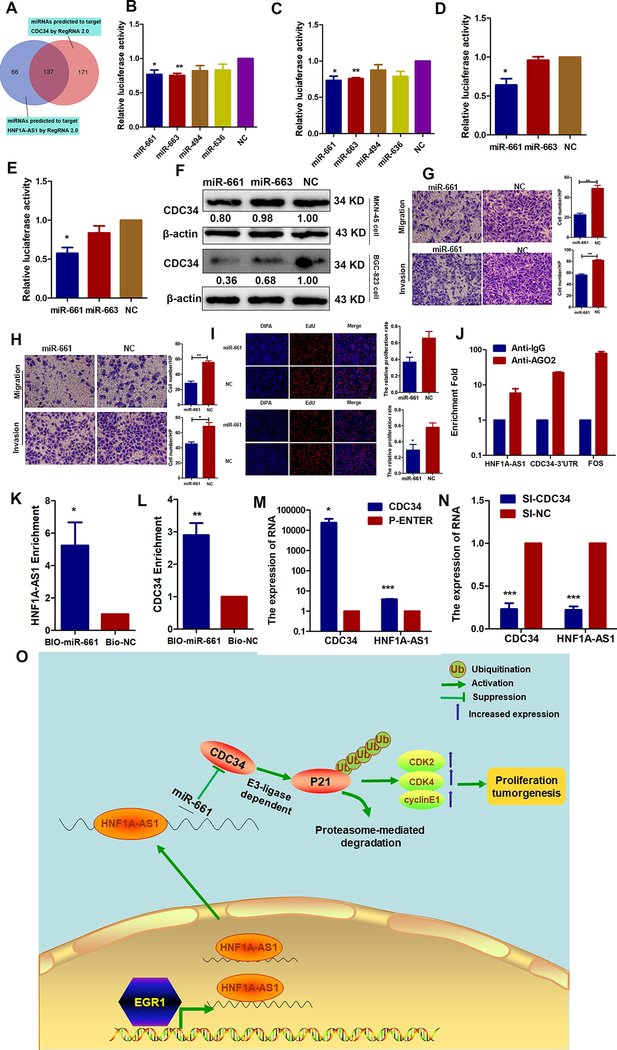
Figure 7. HNF1A-AS1 functions as a ceRNA with miR-661 to upregulate CDC34 expression.
(A). A Venn diagram depicting 137 miRNAs that predicted to target both HNF1A-AS1 and CDC34 by RegRNA 2.0 algorithms.
(B-C). Luciferase activity was detected using the dual-luciferase assay. The data indicated a decrease in luciferase activity in MKN-45(B) and BGC-823 (C) cells transfected with PGLO-HNF1A-AS1 and miR-661 or miR-663.
(D-E). The results indicated a decrease in luciferase activity in MKN-45 (D) and BGC-823 (E) cells transfected with PGLO-CDC34–3’UTR and miR-661.
(F). Western blot analyses were performed to confirm the CDC34 gene expression in both cells transfected with miR-661 or miR-663 mimics.
(G-H). Effects of the miR-661 overexpression on migration and invasion abilities in MKN-45 cells (G) and BGC-823 cells (H).
(I). EdU assays were performed to detect the effects of miR-661 on cell proliferation in MKN-45 cells (up) and BGC-823 cells (bottom).
(J). RNA immunoprecipitation with an anti-Ago2 antibody was used to assess endogenous Ago2 binding to HNF1A-AS1 and CDC-34 3’UTR; IgG was used as the control.
(K-L). BGC-823 cells were transfected with biotinylated miR-661(BIO-miR-661) or biotinylated NC (BIO-NC). Forty-eight hours after transfection, cells were collected for a biotin-based pulldown assay. HNF1A-AS1 and CDC34 expression levels were analyzed by RT–qPCR.
(M-N). RT-qPCR assay was performed to detect HNF1A-AS1 expression in CDC34 up- or down-regulating BGC-823 cells.
(O). Proposed functional action of HNF1A-AS1 in modulating gastric cancer tumorigenesis. The transcriptional factor EGR1 activated HNF1A-AS1 expression by directly binding to its promoter. And HNF1A-AS1 functioned as ceRNA by competitively binding to miR-661 to upregulate the expression of CDC34, which is a direct target of miR-661. Then, CDC34-mediated ubiquitination decreased the stability of p21 protein via degradation of P21 protein, and subsequently increased the expressions of CDK2, CDK4, cyclinE1 to enhance the proliferation capabilities of GC cells.