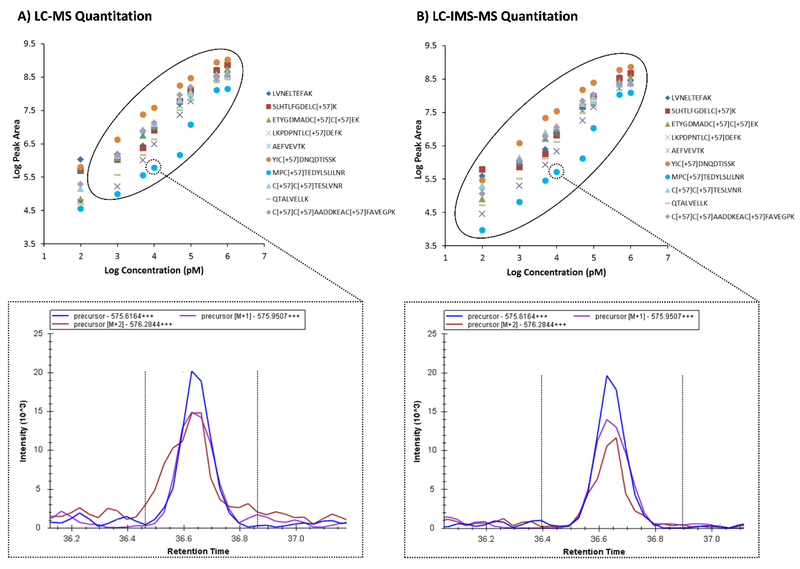
Figure 2.
Addition of the IMS dimension enables better quantitation of MS1 data. Calibration curves for 10 of the BSA target peptides spiked into yeast and extracted in the MS1 spectra either A) without or B) with IMS filtering. IMS filtering illustrated a greater dynamic range for all peptides due to the reduction of noise in the monoisotopic and subsequent isotopic peaks. A specific example of interference is illustrated in the bottom XICs for the second isotope of MPCTEDYSLILNR3+ in the 10 nM BSA/yeast sample, where the red peak appears to have a shoulder at an earlier retention time when IMS filtering was not applied.