Abstract
PURPOSE
To examine prevalence and predictors of social difficulties in adolescent survivors of central nervous system (CNS) tumors.
METHODS
CNS tumor survivors (N=665; 53.8% male; current median age[range] 15.0[12.0-17.0] years; 12.1[8.0-17.7] years from diagnosis; 51.7% treated with cranial radiation[CRT]) were compared to 1376 solid tumor survivors (50.4% male; 15.0[12.0-17.0] years; 13.2[8.3-17.9] years from diagnosis) and 726 siblings (52.2% male; 15.0[12.0-17.0] years). Social adjustment was measured using parent-proxy responses to the Behavior Problems Index. Latent profile analysis (LPA) defined social classes. Multinomial logistic regression adjusting for age, sex, and age at diagnosis identified predictors of class membership. Path analyses tested mediating effects of physical limitations, sensory loss and cognitive impairment on social outcomes.
RESULTS
Caregivers reported CNS tumor survivors had zero friends (15.3%), and interacted with friends less than once/week (41.0%) compared to solid tumor survivors (2.9%, 13.6%) or siblings (2.3%, 8.7%). LPA identified three social classes for CNS tumor survivors: Well-Adjusted (53.4%); Social Deficits (30.4%); and Poor Peer Relationships (16.2%) compared to two classes: Well-Adjusted (86.2%; 91.1%); and Social Deficits (13.8%; 8.9%) for solid tumor survivors and siblings. CRT predicted class membership for CNS survivors (Poor Peer Relationships Odds Ratio[OR] 1.16/10Gy, 95% CI 1.08-1.25; Social Deficits OR 1.14/10Gy, 95% CI, 1.04-1.25; referenced to Well-Adjusted). Cognitive impairment mediated the association between all social outcomes and CRT (p’s<0.001).
CONCLUSION
Almost 50% of CNS tumor survivors experience social difficulties; the pattern is unique compared to solid tumor and sibling groups. Cognitive impairment is associated with increased risk highlighting the need for multi-targeted interventions.
Keywords: Brain Neoplasms, Adolescent, Survivors, Social Adjustment, Cognition
INTRODUCTION
Improved therapies for pediatric central nervous system (CNS) tumors have increased survival rates from 59% between 1975 and 1979 to 75% between 2003 and 20091; there are now more than 115,000 survivors of pediatric CNS tumors living in North America.1 However, survival is not without consequences as many survivors experience significant long-term functional limitations. As adults, survivors are more likely than siblings to require special education services and experience dysfunctional intimate relationships, and are less likely to attend college, to live independently, and be employed.2 Survivors of pediatric CNS tumors also experience deficits in social adjustment (i.e., the ability to achieve personal goals in social interactions while maintaining positive relationships with others over time and across situations3)4 that worsen with time,5 and negatively affect survivors’ long-term quality of life.6
Research examining social adjustment among survivors of pediatric CNS tumors has been limited, reflecting the experiences of survivors from small, single center studies.5,7,8 Moreover, the examination of social adjustment in survivors of pediatric CNS tumors has been superficial, relying on haphazard definitions of the construct and using a highly variable range of paper-and pencil measures.7 The ‘Social Competence Model’ provides a theoretical framework for examining the etiology of social adjustment deficits in children with acquired brain injuries.3 The model takes a multilevel approach to understanding social competence distinguishing among three key levels: 1) social information processing; 2) social interaction; and 3) social adjustment, with social adjustment at the top of the hierarchy. Moreover, it relates insult-related risk factors and social information processing to social interaction and social adjustment deficits. Based on this theoretical model, we speculate that treatment modalities, socio-demographic indicators, and impairments subsequent to tumor control may influence social adjustment. Potential risk factors include cranial radiation therapy (CRT), a known risk factor for cognitive impairment;9,7,10,11 with some evidence linking CRT to social outcomes in medulloblastoma and posterior fossa tumors.12,13 In addition, younger age at diagnosis, male sex and lower socioeconomic status have been associated with poorer social adjustment outcomes in children with traumatic brain injury.14,15 Finally, cognitive impairment, sensory loss or physical impairments often seen in survivors of pediatric CNS tumors may also contribute to social adjustment outcomes.2,16,17 What is missing from the current literature is a comprehensive examination of these risk factors in a large sample of survivors of pediatric CNS tumors.
Thus, the aims of the current study were to: 1) examine patterns of social adjustment (e.g., number of close friends, frequency of interactions, quality of interactions, social withdrawal, conflict) in adolescent survivors of pediatric CNS tumors compared to survivors of neuroblastoma and Wilms tumor (i.e., solid tumors) and to a sibling control group; 2) identify demographic, socioeconomic, disease, and treatment predictors of social adjustment; and 3) examine associations between physical limitations, cognitive impairments and sensory loss and social adjustment.
METHODS
Participants
The Childhood Cancer Survivor Study (CCSS) is a multi-institutional study of 5+year survivors of childhood cancer diagnosed when younger than 21 years of age.18,19 Survivors were treated at one of 31 institutions between 1970 and 1999 for leukemia, Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, neuroblastoma, soft tissue sarcoma, bone cancer, Wilms tumor, or CNS cancer. Study participants completed a baseline questionnaire more than five years post-diagnosis. Information regarding primary cancer diagnosis and treatment was abstracted from medical records at each treating institution. Local institutional review boards approved study procedures, and parental informed consent was obtained for all participants younger than age 18 years.
Participants for the current study were between 12-17 years old at the baseline survey, and were survivors of CNS tumors. Two comparison groups included survivors of neuroblastoma or Wilms (solid) tumors, and siblings of cancer survivors. Survivors of neuroblastoma or Wilms tumor were excluded from analyses if they had experienced a secondary malignancy to the CNS. Among 32,805 survivors from the CCSS combined cohort, 794 were eligible survivors of CNS tumor and 1,445 were eligible survivors of solid tumors (see Figure 1a). 129 (16%) survivors of CNS tumors and 69 (5%) survivors of solid tumors did not complete the social adjustment questionnaires, leaving 665 survivors of CNS tumors and 1376 survivors of solid tumors available for analyses for comparison with 726 siblings (see Figure 1b).
Figure 1.
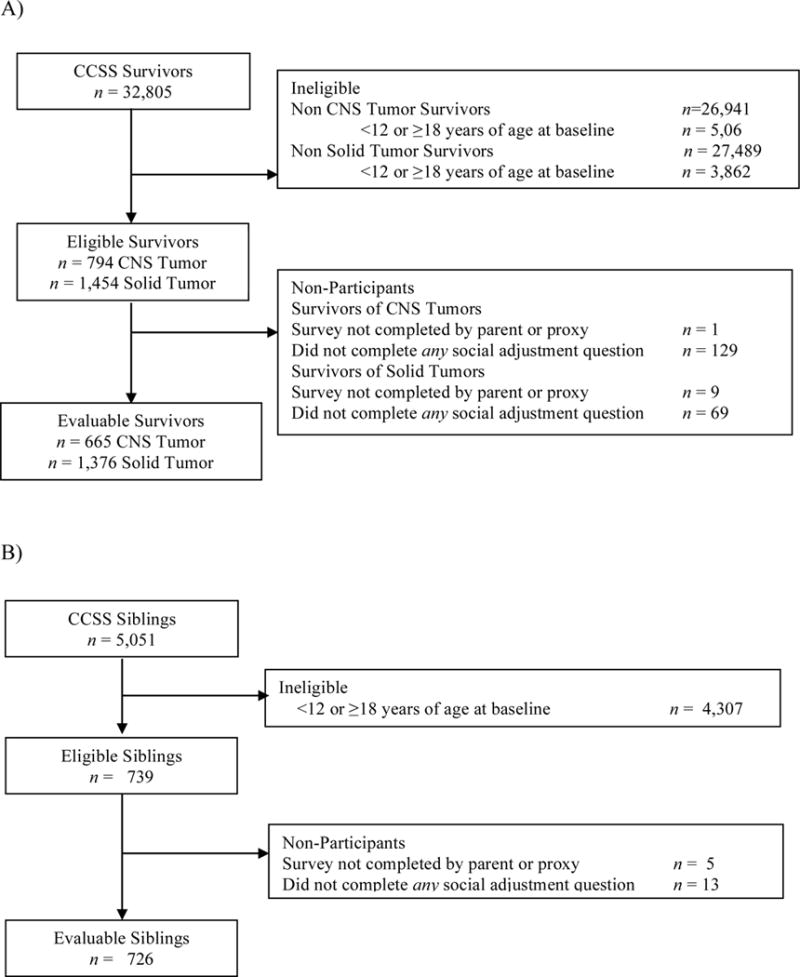
CONSORT diagram of (A) adolescent survivor study participation and (B) sibling participation. CCSS, Childhood Cancer Survivor Study; CNS, Central Nervous System.
Social Adjustment
The primary outcome was parent-proxy reports of social adjustment measured by the Behavior Problems Index (BPI).20 The BPI has been included as a component of the CCSS survey and was originally developed for the National Health Survey by taking a subset of questions from the Child Behavior Checklist21 and includes the following items/subscales related to social adjustment: number of close friends, frequency of interactions, quality of interactions, social withdrawal (e.g., “is not liked by other children”), and antisocial behavior (e.g., “bullies, or is cruel or mean to others”). Social withdrawal and antisocial subscales were rated on a three-point Likert scale ranging from “not true” to “often true”. All ratings were completed by parents or guardians of the adolescents. Additional items were included for Number of Close Friends (i.e., About how many close friends does your child have: 0; 1; 2-3; 4 or more), Frequency of Interactions (i.e., About how many times a week does your child do things with close friends: Less than 1; 1 or 2; 3 or more), and Quality of Interactions (i.e., Compared to other children of his/her age, how well does your child: Get along with his/her brothers and sisters; Get along with other children; Behave with his/her parents; play and work by himself/herself: Better; About the Same; Worse). Where necessary, social adjustment items were recoded so that higher scores consistently represented more problems (i.e., fewer close friends; fewer interactions).
Treatment Exposures and Covariates
Demographic, socioeconomic, disease and treatment variables were considered as potential predictors of social function. These included sex, current age, household income (i.e., <$40,000, $40,000-$80,000, >$80,000), family size (i.e., 0/1 siblings, 2 or more siblings), tumor diagnosis (i.e., astrocytoma, medulloblastoma, other CNS), age at diagnosis, decade of diagnosis (1970-1989, 1990-1999) and treatment (i.e., CRT dose). Using data from a detailed review of radiation therapy records, maximum prescribed dose was reconstructed (using previously described methods)22 to one of four segments of the brain including: 1) posterior fossa; 2) temporal lobes; 3) frontal lobes; and 4) parietal/occipital lobes.
We considered potential mediating effects of physical limitations, cognitive impairments and sensory loss on social adjustment. Physical limitations were assessed with the following items derived from the SF-3623 physical limitations subscale and included the following items: vigorous activities, moderate activities, walking uphill or climbing stairs, bending or stooping, walking one block, eating or personal hygiene (each rated on a likert scale as “not limited”, “limited for ≤ 3 months” or “limited for > 3 months”). Cognitive impairment was operationalized as “yes” or “no” based on history of learning or concentration problems requiring special education services. Sensory loss was operationalized as “yes” or “no” based on responses to questions targeting hearing and/or vision loss.
Statistical Analysis
Demographic characteristics were summarized and compared between survivors and siblings using t-tests or chi-square where appropriate. Latent profile analysis (LPA) was used to identify social classes based on item level responses to the BPI for each group separately (i.e., survivors CNS tumors, survivors of solid tumors and siblings). The number of classes was not pre-set. However, a minimum class size of 5% of the sample was used as a threshold. Multivariable multinomial logistic regression analyses were conducted to identify demographic, socioeconomic, disease and treatment predictors related to the three social classes identified among survivors of CNS tumors. Analyses were conducted separately for diagnosis and treatment to avoid confounding. In addition, we were keen to distinguish between the differing contributions of diagnosis versus treatment exposures in contributing to social adjustment difficulties. Predictors included tumor diagnosis, radiation dosimetry, age at diagnosis, sex, age at survey, household income, and decade of diagnosis (1970-1989, 1990-1999). Path analyses were conducted to examine the mediating effects of physical limitations, cognitive impairment and sensory loss between treatment factors and each of the five social adjustment outcomes. Analyses began with a proposed theoretical model of how these limitations might mediate the association between treatment and social adjustment outcomes. Paths with a modification index of 3.6 or higher and a meaningful clinical interpretation were added to the model one at a time. After all suggested paths were added, the model was modified based on the following criteria: 1) paths with absolute value of standardized coefficient < 0.05 were removed, one path at a time, beginning with the path that has the smallest absolute value of standardized coefficient; and 2) paths that had a P>0.05 were removed, one path at a time, beginning with the path that had the largest P-value. The target fitting criteria included: Comparative fit index (CFI) and Tucker Lewis Index (TLI) >0.95; Root Mean Square Error of Approximation (RMSEA) <0.05. Theta parameterization was used because some exogenous variables were categorical. All analyses were completed using Mplus v7.11 or SAS v9.4.
Results
Missing Data
A higher percentage of survivors of CNS tumors (n=129, 16%) compared to survivors of solid tumors (n=69, 5%) or siblings (n=13, 2%) did not have data on social adjustment questions. Given the high rate of non-respondents for survivors of CNS tumors, we compared respondents to non-respondents; those who did not complete the questions were treated with higher doses of cranial radiation (p’s < .001), were older at diagnosis (p < .001) and had longer time since diagnosis (p < .001) compared to those who completed the social adjustment questions (see Supplemental Table A1).
Descriptive Characteristics of the Sample
Characteristics of survivors are shown in Table 1. The most common CNS tumor diagnosis was Astrocytoma (57.4%) followed by Medulloblastoma (23.5%). Overall, the total mean CRT dose for survivors of CNS tumors was 27.0 Gy (SD = 26.6 Gy). Means and standard deviations for proxy-reported social adjustment outcomes as well as frequencies for each response are shown in Table 2. Importantly, based on proxy-reports, survivors of CNS tumors scored significantly worse on all social adjustment outcomes compared to survivors of solid tumors and siblings with the exception of antisocial behavior which did not significantly differ among childhood cancer survivors (CNS and solid tumor). Nearly seven times as many survivors of CNS tumors (15.3%) reported zero friends compared to survivors of solid tumors (2.9%) and siblings (2.3%). In addition, caregivers reported survivors of CNS tumors interacted with friends less than once per week (41.0%) compared to survivors of solid tumors (13.6%) and siblings (8.7%).
Table 1.
Demographic and treatment characteristics of survivors of CNS tumors, solid tumors and siblings
| Characteristic | CNS Tumor (n=665) | Solid Tumor (n=1376) | Sibling (n=726) | Pa | Pb | Pc | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | Median (Range) | N (%) | Median (Range) | N (%) | Median (Range) | |||||
| Sex | Male | 358 (53.8) | 694 (50.4) | 379 (52.2) | .15 | .54 | .44 | |||
| Female | 307 (46.2) | 682 (49.6) | 347 (47.8) | |||||||
| Age (Year) | 15.0 (12.0-17.0) | 15.0 (12.0-17.0) | 15.0 (12.0-17.0) | |||||||
| Household Income | < 40,000 | 161 (25.8) | 331 (25.9) | 133 (19.3) | .74 | .01 | <.001 | |||
| 40,000-79,999 | 199 (31.9) | 427 (33.4) | 223 (32.3) | |||||||
| >=80,000 | 264 (42.3) | 519 (40.6) | 334 (48.4) | |||||||
| Race/Ethnicity | White | 555 (87.0) | 1099 (82.3) | 600 (86.3) | .009 | .56 | <.001 | |||
| Black | 31 (4.9) | 122 (9.1) | 28 (4.0) | |||||||
| Hispanic | 35 (5.5) | 72 (5.4) | 40 (5.8) | |||||||
| Other | 17 (2.7) | 42 (3.1) | 27 (3.9) | |||||||
| Diagnosis | Astrocytoma | 382 (57.4) | ||||||||
| Medulloblastoma, | 156 (23.5) | |||||||||
| PNET | ||||||||||
| Other CNS tumors | 127 (19.1) | |||||||||
| Neuroblastoma | 703 (51.1) | |||||||||
| Wilms Tumor | 673 (48.9) | |||||||||
| No cancer | 726 | |||||||||
| Age at Diagnosis (Year) | 2.9 (0.0-9.2) | 1.5 (0.0-8.4) | ||||||||
| Time Since Diagnosis | 12.1 (8/0- | 13.2 (8.3- | ||||||||
| (Year) | 17.7) | 17.9) | ||||||||
| CRT | No | 291 (48.3) | 1192 (95.8) | 726 | <.0001 | |||||
| Yes | 312 (51.7) | 52 (4.2) | ||||||||
| CRT Dose (Gy) | Mean (SD) | |||||||||
| Posterior Fossa | 20.3 (25.3) | |||||||||
| Temporal Lobe | 23.0 (25.1) | |||||||||
| Frontal Cortex | 11.7 (18.0) | |||||||||
| Parietal or Occipital | 11.2 (17.6) | |||||||||
| Lobe | ||||||||||
| Total Dose | 27.0 (26.6) | |||||||||
| Decade of Diagnosis | 1970-1989 | 367 (55.94) | ||||||||
| 1990-1999 | 293 (44.06) | |||||||||
| SMN | No | 654 (98.3) | 1355 (98.5) | 726 | 0.83 | |||||
| Yes | 11 (1.7) | 21 (1.5) | ||||||||
| Relapse | No | 650 (97.7) | 1372 (99.7) | 726 | <.0001 | |||||
| Yes | 15 (2.3) | 4 (0.3) | ||||||||
Abbreviations: CNS, central nervous system; CRT, cranial radiation therapy; SMN, secondary malignancy.
CNS tumor vs.Solid tumor;
CNS tumor vs. Sibling controls;
Solid tumor vs. Sibling controls
Table 2.
Social adjustment outcomes among survivors of childhood CNS tumors, solid tumors and siblings
| CNS | Solid Tumor | Siblings | Pa | Pb | Pc | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Scale | Response | N (%) | M (SD) | N (%) | M (SD) | N (%) | M (SD) | |||
| Number of Friends | 1.0-4.0 | 2.1 (1.0) | 1.5 (0.7) | 1.6 (0.7) | <.001 | <.001 | |||||
| 0 | 100 (15.3) | 40 (2.9) | 16 (2.3) | <.001 | <.001 | .23 | |||||
| 1 | 88 (13.5) | 79 (5.8) | 53 (7.5) | ||||||||
| 2-3 | 252 (38.6) | 437 (32.2) | 245 (34.5) | ||||||||
| 4 or more | 213 (32.6) | 800 (59.0) | 397 (55.8) | ||||||||
| Time With Friends | 1.0-3.0 | 2.1 (0.9) | 1.6 (0.7) | 1.5 (0.7) | <.001 | <.001 | .001 | ||||
| Less than 1/week | 269 (41.0) | 184 (13.6) | 62 (8.7) | <.001 | <.001 | .003 | |||||
| 1 or 2/week | 176 (26.8 | 487 (35.9) | 255 (35.7) | ||||||||
| 3 or more/week | 211 (32.2) | 686 (50.6) | 398 (55.7) | ||||||||
| Quality of Interactions (compared to others) | 4.0-12.0 | 7.2 (1.8) | 6.7 (1.7) | 6.6 (1.7) | <.001 | <.001 | .05 | ||||
| Get along with brothers and sisters | |||||||||||
| Better | 176 (26.7) | 401 (29.3) | 235 (32.6) | .002 | .005 | .23 | |||||
| About the Same | 425 (64.5) | 902 (65.9) | 448 (62.1) | ||||||||
| Worse | 58 (8.8) | 66 (4.8) | 38 (5.3) | ||||||||
| Get along with others | |||||||||||
| Better | 150 (22.8) | 489 (35.7) | 282 (39.2) | <.001 | <.001 | .055 | |||||
| About the Same | 396 (60.2) | 813 (59.4) | 417 (57.9) | ||||||||
| Worse | 112 (17.0) | 66 (4.8) | 21 (2.9) | ||||||||
| Behave with parents | |||||||||||
| Better | 254 (38.6) | 553 (40.4) | 310 (43.1) | .23 | .047 | .43 | |||||
| About the Same | 355 (60.2) | 739 (54.0) | 376 (52.2) | ||||||||
| Worse | 112 (17.0) | 76 (5.6) | 34 (4.7) | ||||||||
| Plays alone | |||||||||||
| Better | 257 (39.1) | 566 (41.3) | 321 (44.6) | <.001 | <.001 | .36 | |||||
| About the Same | 332 (50.5) | 730 (53.3) | 362 (50.3) | ||||||||
| Worse | 69 (10.5) | 73 (5.3) | 37 (5.1) | ||||||||
| Social Withdrawal | 3.0-9.0 | 4.3 (1.6) | 3.6 (1.2) | 3.4 (1.0) | <.001 | <.001 | <.001 | ||||
| Trouble getting along with other children | |||||||||||
| Not true | 449 (67.9) | 1129 (82.2) | 647 (89.4) | <.001 | <.001 | <.001 | |||||
| Sometimes true | 149 (22.5) | 203 (14.8) | 66 (9.1) | ||||||||
| Often True | 63 (9.5) | 41 (3.0) | 11 (1.5) | ||||||||
| Not liked by other children | |||||||||||
| Not true | 425 (64.3) | 1140 (83.1) | 647 (89.4) | <.001 | <.001 | <.001 | |||||
| Sometimes true | (181 (27.4) | 202 (14.7) | 64(8.8) | ||||||||
| Often True | 55 (8.3) | 30 (2.2) | 13 (1.8) | ||||||||
| Withdrawn | |||||||||||
| Not true | 409 (61.9) | 1109 (80.8) | 624 (86.2) | <.001 | <.001 | .007 | |||||
| Sometimes true | 196 (29.7) | 217 (15.8) | 84 (11.6) | ||||||||
| Often True | 56 (8.5) | 47 (3.4) | 16 (2.2) | ||||||||
| Antisocial | 5.0-15.0 | 6.4 (1.9) | 6.4 (2.0) | 6.1 (1.8) | .52 | .005 | .01 | ||||
| Cheats | |||||||||||
| Not true | 457 (68.9) | 977 (71.2) | 528 (72.7) | .18 | .22 | .049 | |||||
| Sometimes true | 180 (27.1) | 327 (23.8) | 178 (24.5) | ||||||||
| Often True | 26 (3.9) | 69 (5.0) | 20 (2.8) | ||||||||
| Bullies | |||||||||||
| Not true | 553 (83.4) | 1169 (85.1) | 644 (88.7) | .33 | .015 | .062 | |||||
| Sometimes true | 95 (14.3) | 167 (12.2) | 69 (9.5) | ||||||||
| Often True | 15 (2.3) | 38 (2.8) | 13 (1.8) | ||||||||
| Does not feel sorry | |||||||||||
| Not true | 508 (76.6) | 1067 (77.7) | 606 (83.5) | .51 | .006 | .006 | |||||
| Sometimes true | 125 (18.9) | 234 (17.0) | 96 (13.2) | ||||||||
| Often True | 30 (4.5) | 72 (5.2) | 24 (3.3) | ||||||||
| Is disobedient | |||||||||||
| Not true | 422 (63.7) | 900 (65.5) | 498 (68.6) | .71 | .15 | .35 | |||||
| Sometimes true | 208 (31.4) | 409 (29.8) | 198 (27.3) | ||||||||
| Often True | 33 (5.0) | 65 (4.7) | 30 (4.1) | ||||||||
| Trouble getting along with teachers | |||||||||||
| Not true | 550 (83.0) | 1162 (84.6) | 626 (86.2) | .39 | .18 | .59 | |||||
| Sometimes true | 100 (15.1) | 179 (13.0) | 85 (11.7) | ||||||||
| Often True | 13 (2.0) | 33 (2.4) | 15 (2.1) | ||||||||
CNS tumor vs. Solid tumor;
CNS tumor vs. Sibling controls;
Solid tumor vs. Sibling control
Profile Analysis
Results of the LPA yielded three clinically relevant profiles for the CNS survivor group, including “well-adjusted” (53.4%), “social adjustment deficits” (16.2%) and a third class (30.4%) that included those with fewer number of friends and time spent with friends “poor peer relationships”. For analyses conducted for survivors of solid tumors and siblings, separately, both revealed only a two-factor solution including “well adjusted” (86.2%, 91.1%, respectively, Figure 2) and “social adjustment deficits” (13.8%, 8.9%, respectively). Model fit statistics for each latent profile analysis can be found in Supplemental Table A2 (online only).
Figure 2.
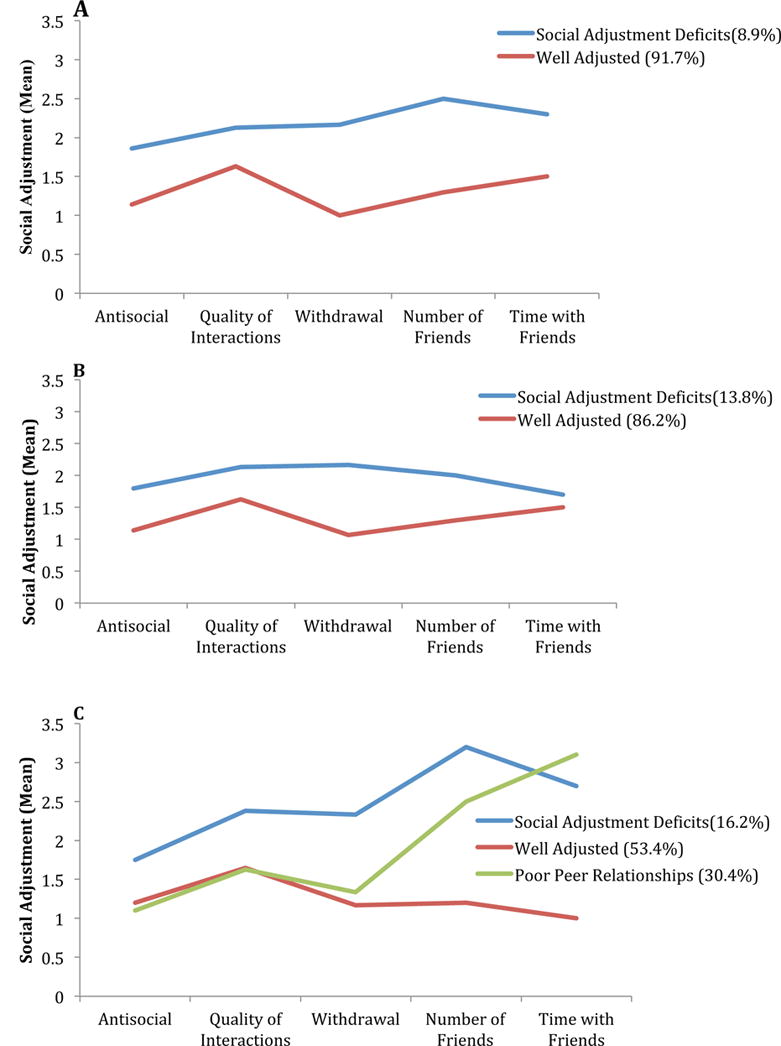
Social adjustment profiles for (A) siblings, (B) survivors of solid tumors and (C) survivors of CNS tumors.
Factors Related to Social Adjustment
Results of multivariable regression analyses specific to survivors of CNS tumors revealed CRT dose exposure was a significant predictor of class membership (Poor Peer Relationships OR 1.12 per 10 Gy increase, 95% CI 1.08 to 1.25; Social Adjustment Deficits OR 1.14 per 10 Gy increase, 95% CI 1.04 to 1.25; compared to Well-Adjusted group, Table 3). The risk of having Social Adjustment Deficits or Poor Peer Relationships increased with CRT dose. Decade of diagnosis was also a significant predictor of class membership. Specifically, the 1990-99 decade was more likely to be in the poor peer relationship class than in the well-adjusted class (OR 1.67, 95% CI 1.10-2.54).
Table 3.
Variables Associated With Latent Class Membership in CNS Tumor Survivors
| Poor Peer Relationships vs. Well Adjusted | Social Adjustment Deficits vs. Well Adjusted | |||
|---|---|---|---|---|
| Variable | OR | 95% CI | OR | 95% CI |
| Model 1: Diagnosis | ||||
| Age (per year increase) | ||||
| At Baseline | 1.05 | 0.93-1.18 | 0.91 | 0.79-1.05 |
| At Diagnosis | 0.91 | 0.82-1.01 | 0.98 | 0.86-1.11 |
| Sex | ||||
| Male | 0.90 | 0.62-1.30 | 1.17 | 0.74-1.86 |
| Female | 1.00 | 1.00 | ||
| Income | ||||
| <$40,000 | 1.32 | 0.84-2.07 | 1.23 | 0.70-2.18 |
| $40,000-$79,999 | 1.10 | 0.71-1.69 | 1.13 | 0.66-1.93 |
| >$80,000 | 1.00 | |||
| Diagnosis | ||||
| Astrocytoma | 1.04 | 0.64-1.69 | 1.42 | 0.76-2.67 |
| Medulloblastoma, PNET | 1.71 | 0.98-2.98 | 1.61 | 0.77-3.37 |
| Other CNS Tumors | 1.00 | 1.00 | ||
| Decade of Diagnosis | ||||
| 1970-1989 | 1.00 | 1.00 | ||
| 1990-1999 | 1.31 | 0.89-1.92 | 0.94 | 0.57-1.54 |
| Model 2: Treatment | ||||
| Age (per year increase) | ||||
| At Baseline | 1.07 | 0.94-1.21 | 0.95 | 0.81-1.10 |
| At Diagnosis | 0.90 | 0.80-1.00 | 0.96 | 0.84-1.11 |
| Sex | ||||
| Male | 0.88 | 0.59-1.29 | 1.17 | 0.72-1.89 |
| Female | 1.00 | 1.00 | ||
| Income | ||||
| <$40,000 | 1.19 | 0.73-1.93 | 1.38 | 0.77-2.48 |
| $40,000-$79,999 | 1.09 | 0.69-1.71 | 1.10 | 0.63-1.93 |
| >$80,000 | 1.00 | 1.00 | ||
| CRT (per 10 Gy increase) | ||||
| 1.16 | 1.08-1.25 | 1.14 | 1.04-1.25 | |
| Decade of Diagnosis | ||||
| 1970-1989 | 1.00 | |||
| 1990-1999 | 1.67 | 1.10-2.54 | 1.03 | 0.62-1.74 |
Abbreviations: CRT, cranial radiation therapy.
Path Analysis
Final models were on average 8 paths [range 7 to 10] different than the original proposed theoretical model. Cognitive impairment mediated the association between CRT and quality of interactions (standardized β=0.36, p<.001, Figure 3, Supplemental Table A3) and social withdrawal (standardized β=0.29, p<.001). Cognitive impairment similarly mediated the association between physical limitations and number of friends (standardized β=0.38, p<.001), time with friends (standardized β=0.27, p<.001), quality of interactions (standardized β=0.36, p<.001), social withdrawal (standardized β=0.29, p<.001) and antisocial behavior (standardized β=0.19, p<.001).
Figure 3.
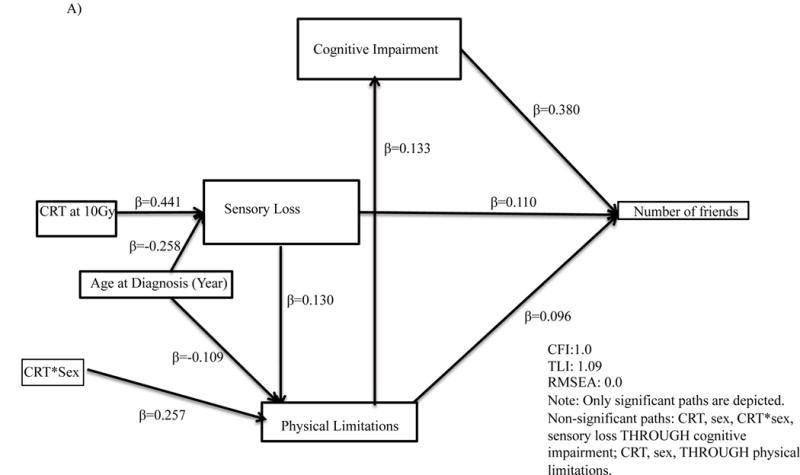
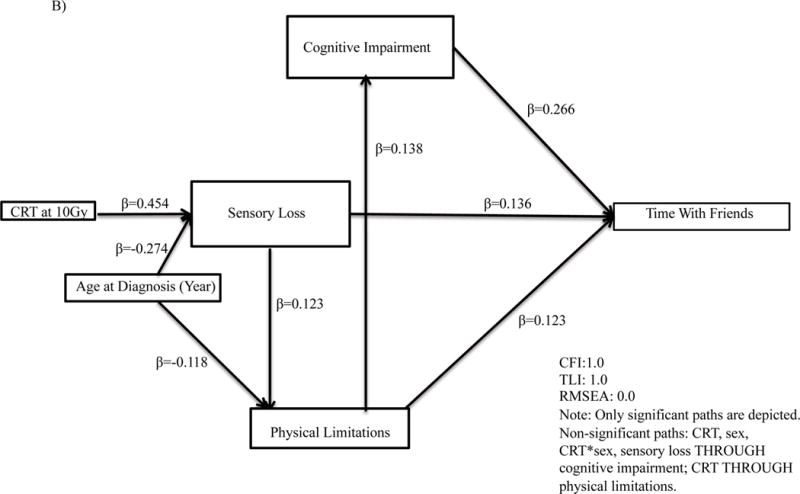
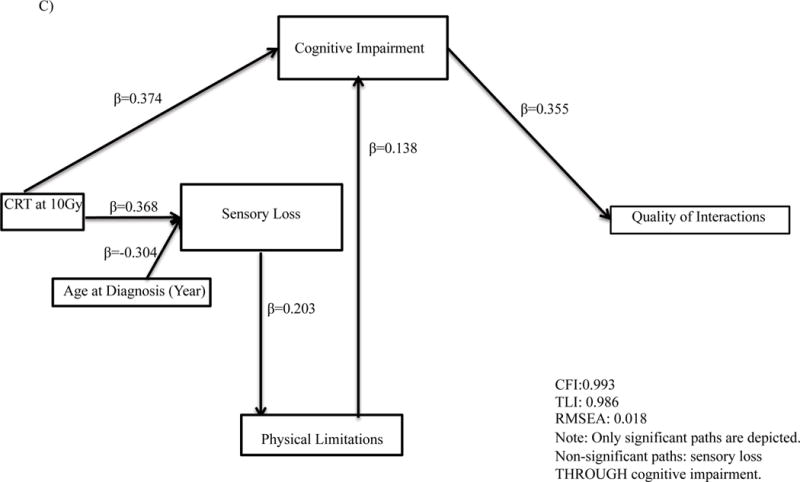
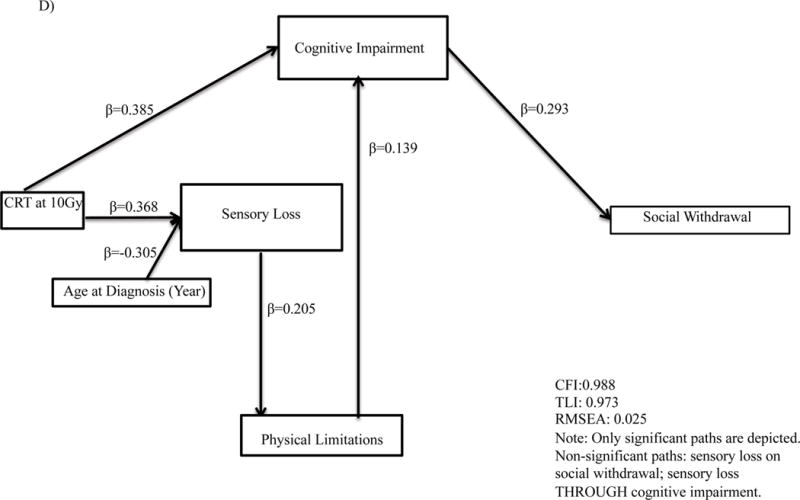
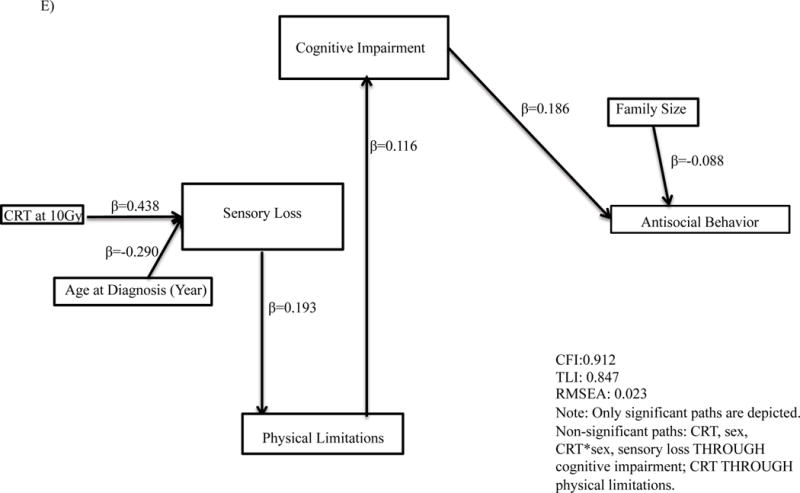
Path analyses for associations for (A) number of friends; (B) time with friends; (C) quality of interactions; (D) social withdrawal; (E) antisocial behavior. CRT, Cranial Radiation Therapy.
Discussion
Results of this study revealed that survivors of CNS tumors demonstrated quantitatively different patterns of social adjustment compared to survivors of non-CNS solid tumors and siblings. Nearly 50% of survivors of CNS tumors had patterns of social behaviors reflecting social adjustment deficits and poor peer relations. CRT dose exposure was significantly associated with these adverse social profiles, though these associations were mediated by symptoms of cognitive impairment. While our findings support previously established risk factors of poor social outcomes, we identified distinct profiles of social adjustment among survivors of CNS tumors that may necessitate different intervention approaches.
Results of our study revealed that CRT was the only significant predictor of class membership in survivors of CNS tumors. Although CRT has been identified as the exposure associated with neurocognitive impairment among this population,9 less has been known about the impact of CRT on social adjustment outcomes. This is one of the few studies that has demonstrated a direct link between CRT and social adjustment deficits among a large heterogeneous sample of survivors of CNS tumors. There has been some evidence of this relationship demonstrated previously in the literature, however, this research has either focused on the intensity of CNS treatments as a whole (chemotherapy included)24 or was focused on specific tumor diagnoses, namely, medulloblastoma or posterior fossa tumors.12,13 Isolating CRT as a significant predictor of social class is important as it highlights the deleterious impact of CNS-directed treatment on social adjustment over and above other treatment, tumor, demographic and socioeconomic variables.11
The relationship among variables revealed more complex interactions based on path analyses. Consistent across all path analyses, cognitive impairment emerged as a significant mediator of social outcomes. Although there is considerable evidence documenting the presence of cognitive impairment in survivors of pediatric CNS tumors, few studies have simultaneously explored relationships between cognitive impairment and social adjustment. Where work has been conducted, significant positive relationships have been identified whereby greater cognitive impairment has been associated with greater social adjustment difficulties.25,26 Cognitive impairment would be expected to have pervasive effects on children’s perception and interpretation of social situations and behavioral responses in social interactions.27 For example, children with cognitive-executive deficits may have difficulty thinking about multiple social-perspectives or response options when determining how to respond to social stimuli. Yet, to date, behavioral interventions targeting social adjustment in pediatric brain tumor survivors may have failed to address the impact of cognitive difficulties in social interactions.28-30 There have been a number of attempts to develop interventions targeting the cognitive difficulties of this population.31-33 To date, these interventions have been met with variable success. While social-cognitive interventions do exist, these have generally been trialed in adult patients with clinical populations including schizophrenia, autism spectrum disorder, or acquired brain injury.34 Future research could work to adapt these interventions for a pediatric CNS tumor population.
Interestingly, physical limitations consistently had an indirect effect on social adjustment mediated by cognitive impairment. Physical limitations have been associated with social adjustment difficulties among this population.17,35 Specifically, adult survivors of pediatric CNS tumors have been found to have more physical limitations when compared to healthy controls and to avoid aspects of their physical (e.g., going to unfamiliar places) and social (e.g., going to a friend’s home) environments. Physical limitations have also been linked to poorer social functioning (e.g., high-school graduation, employment, relationship outcomes).2 That cognitive impairments mediated the effect of physical limitations on social adjustment may reflect those patients who have received the most intense treatments thereby impacting multiple functional domains. Physical limitations and sensory loss also had a direct role in affecting social adjustment when it came to the number of close friends or the time spent with friends. This is not surprising as these limitations would be expected to interfere with a survivors’ ability to interact with their peers. Other noteworthy relationships based on path analyses revealed that in males, CRT dose did not have any impact on physical limitations whereas in females an increasing CRT dose was associated with increased physical limitations. Females have tended to show inferior outcomes across a wide variety of late effects including cognitive deficits following CRT, cardiovascular outcomes, obesity, and risk of osteonecrosis suggesting there may be broader biological and physiological underpinnings to these sex-specific differences.36 Future research is needed to test these hypotheses.
Additional factors that have been explored in the context of social adjustment and require some additional discussion include time since diagnosis and age at diagnosis. Time since diagnosis was not found to be significantly related to social adjustment in multinomial logistic regression analyses. This finding is in contrast to existing literature that has suggested social adjustment deficits in survivors of pediatric CNS tumors worsen with time.37,38 Within our sample, time since diagnosis ranged from 8-17 years and therefore the extent of these deficits may have already been realized within this time frame. Time since diagnosis may play a more important role in the more acute post-diagnosis phase (i.e., <8 years post diagnosis). Age at diagnosis was found to be a consistent predictor in path analyses. This finding is consistent with literature that has shown the younger the age of diagnosis and treatment, the worse the functional outcomes.39
Although not the focus of the current analysis, some discussion of the outcomes related to our solid tumor comparison group is warranted. These survivors demonstrated significantly worse social adjustment outcomes with respect to spending time with friends and social withdrawal when compared to sibling controls. There is an extensive body of literature that has documented the social difficulties among survivors of pediatric cancer as a whole2,41,42 as well as more broadly for children with a chronic illness.43 Thus, the findings in this study support the notion that children with chronic illness are not immune from suffering social difficulties. Moreover, neither survivors of solid tumors nor siblings are immune from the psychological effects of childhood cancer.44,45 Consistently, however, survivors of CNS tumors have been found to fare worse with respect to other patient populations.41 In addition, the impact of CRT and cognitive difficulties on social outcomes as revealed in this study, suggest that the mechanisms for social difficulties among survivors of CNS tumors may be different from those of other diagnoses. The underlying mechanisms (i.e., neuropsychological deficits, neurological or structural changes) for social difficulties among survivors of CNS tumors have yet to be elucidated.7 Future research might consider examination of these mechanisms relative to non-cancer affected peer groups.
The current findings support modern theoretical assumptions of social competence in children with acquired brain injuries that purport multilevel, hierarchical models beginning with, social information processing (i.e., social-cognitive processes), followed by social interactions (i.e. peer relations) and finally social adjustment.3 Although these three components are considered to be interrelated, within the theoretical model they are conceptualised as distinct processes. In our sample of survivors of pediatric CNS tumors, peer relations (or lack thereof) emerged as a distinct social class that was not present in survivors of non-CNS tumors or siblings. There has been additional evidence to support application of this theoretical model in survivors of CNS tumors; survivors of pediatric CNS tumors experience deficits at the levels of social information processing, social interactions and social adjustment.8 The relationships among each level, however, and the predictive value of each require further investigation.46,47
There were several limitations with this study. First, responses to the questionnaire were based on parent-proxy reports. Given considerable research that has documented the discrepancies between parent-proxy and self-report particularly as it relates to social adjustment, this study would have benefited from the addition of self-reports of social adjustment. In addition, this study lacked reports of social adjustment from teachers and peers. Peer data is often acknowledged as the gold-standard for documenting social functioning. There was a significant proportion of survivors of CNS tumors for whom the questionnaires were not completed, and the results from this study may not be generalizable to the entire population of survivors of CNS tumors. Parents who did not complete the questionnaires were more likely to have received higher doses of CRT. Thus, current results may underestimate the prevalence of social adjustment problems in this population. Finally, our cross-sectional study design precludes conclusions regarding causation. Future research should aim to study the trajectories of social adjustment from the time of diagnosis through to survivorship to determine whether CRT does indeed predict social adjustment outcomes.
In conclusion, based on our large sample of survivors of CNS tumors, almost 50% of survivors of CNS tumors report patterns of social adjustment difficulties compared to only 14% and 9% in survivors of non-CNS cancers and siblings respectively. Moreover, patterns of social difficulty were unique to these survivors. Predictors of social adjustment difficulties were also unique to survivors of CNS tumors isolating cognitive impairment as a significant mediator of social outcomes over and above other socio-demographic or disease or treatment related factors. There have been multiple efforts to address the cognitive impairments of pediatric brain tumor survivors.48 Recent attempts to improve social adjustment also exist but have been met with small overall effects.28,49,50 Future research should focus on the potential for a combination of cognitive and social remediation strategies to positively impact social adjustment.
Supplementary Material
Acknowledgments
Funding: This work was supported by the National Cancer Institute (CA55727, Armstrong, PI). Support to St. Jude Children’s Research Hospital also provided by the Cancer Center Support (CORE) grant (CA21765, Roberts, PI) and by ALSAC.
Footnotes
Conflicts of Interest: There are no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Fiona Schulte: conceptualization, methodology, formal analysis, visualization, writing – original draft, writing – review and editing
Tara M. Brinkman: methodology, writing – review and editing
Chenghong Li: Data curation, formal analysis, methodology, visualization, writing – review and editing
Taryn Fay-McClymont: writing – review and editing
Deokumar Srivastava: Data curation, formal analysis, methodology, supervision, visualization, writing – review and editing
Kirsten K. Ness: writing – review and editing
Rebecca M. Howell: data curation, writing – review and editing
Sabine Mueller: data curation, writing – review and editing
Elizabeth Wells: data curation, writing – review and editing
Doug Strother: writing – review and editing
Lucie Lafay-Cousin: writing – review and editing
Wendy Leisenring: writing – review and editing
Leslie L. Robison: funding acquisition, investigation, writing – review and editing
Gregory T. Armstrong: funding acquisition, investigation, project administration, resources, writing – review and editing
Kevin R. Krull: conceptualization, data curation, formal analysis, investigation, methodology, resources, visualization, writing – review and editing
References
- 1.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA: a cancer journal for clinicians. 64:83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 2.Gurney JG, Krull KR, Kadan-Lottick N, et al. Social Outcomes in the Childhood Cancer Survivor Study Cohort. Journal of Clinical Oncology. 2009;27(14):2390–2395. doi: 10.1200/JCO.2008.21.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeates KO, Bigler ED, Dennis M, et al. Social outcomes in childhood brain disorder: A heuristic integration of social neuroscience and developmental psychology. Psychological Bulletin. 2007;133:535–556. doi: 10.1037/0033-2909.133.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vannatta K, Garstein MA, Short A, Noll RB. A controlled study of peer relationships of children surviving brain tumors: teacher, peer, and self-ratings. Journal of Pediatric Psychology. 1998;23:279–287. doi: 10.1093/jpepsy/23.5.279. [DOI] [PubMed] [Google Scholar]
- 5.Schulte F, Barrera M. Social Competence in Childhood Brain Tumor Survivors: A Comprehensive Review. Supportive Care Cancer. 2010;18:1499–1513. doi: 10.1007/s00520-010-0963-1. [DOI] [PubMed] [Google Scholar]
- 6.Zebrack BJ, Gurney JG, Oeffinger K, et al. Psychological outcomes in long-term survivors of childhood brain cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2004;22(6):999–1006. doi: 10.1200/JCO.2004.06.148. [DOI] [PubMed] [Google Scholar]
- 7.Schulte F. Social competence in pediatric brain tumor survivors: breadth versus depth. Current opinion in oncology. 2015;27:306–310. doi: 10.1097/CCO.0000000000000193. [DOI] [PubMed] [Google Scholar]
- 8.Hocking MC, McCurdy M. Social competence in pediatric brain tumor survivors: Application of a model from social neuroscience and developmental psychology. Pediatric blood {&} cancer. 2015:375–384. doi: 10.1002/pbc.25300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddick WE, White HA, Glass JO, et al. Developmental model relating white matter volume to neurocognitive deficits in pediatric brain tumor survivors. Cancer. 2003;97:2512–2519. doi: 10.1002/cncr.11355. [DOI] [PubMed] [Google Scholar]
- 10.Bonner MJ, Hardy KK, WIllard VW, Anthony KK, Hood M, Gururangan S. Social functioning and facial expression recognition in survivors of pediatric brain tumors. Journal of Pediatric Psychology. 2008 doi: 10.1093/jpepsy/jsn035. [DOI] [PubMed] [Google Scholar]
- 11.Willard VW, Hardy KK, Bonner MJ. Gender differences in facial expression recognition in survivors of pediatric brain tumors. Psycho-oncology. 2009;18:893–897. doi: 10.1002/pon.1502. [DOI] [PubMed] [Google Scholar]
- 12.Brinkman TM, Palmer SL, Chen S, et al. Parent-reported social outcomes after treatment for pediatric embryonal tumors: a prospective longitudinal study. J Clin Oncol. 2012;30(33):4134–4140. doi: 10.1200/JCO.2011.40.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mabbott DJ, Spiegler BJ, Greenberg ML, Rutka JT, Hyder DJ, Bouffet E. Serial evaluation of academic and behavioral outcome after treatment with cranial radiation in childhood. J Clin Oncol. 2005;23(10):2256–2263. doi: 10.1200/JCO.2005.01.158. [DOI] [PubMed] [Google Scholar]
- 14.Yeates KO, Swift E, Taylor HG, et al. Short- and long-term social outcomes following pediatric traumatic brain injury. Journal of the International Neuropsychological Society. 2004;10(3):412–426. doi: 10.1017/S1355617704103093. [DOI] [PubMed] [Google Scholar]
- 15.Anderson V, Beauchamp MH, Yeates KO, Crossley L, Hearps SJC, Catroppa C. Social competence at 6 months following childhood traumatic brain injury. Journal of the International Neuropsychological Society : JINS. 2013;19:539–550. doi: 10.1017/S1355617712001543. [DOI] [PubMed] [Google Scholar]
- 16.Piscione PJ, Bouffet E, Mabbott DJ, Shams I, Kulkarni AV. Physical functioning in pediatric survivors of childhood posterior fossa brain tumors. Neuro-oncology. 2014;16:147–155. doi: 10.1093/neuonc/not138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brinkman TM, Li Z, Neglia JP, et al. Restricted access to the environment and quality of life in adult survivors of childhood brain tumors. Journal of neuro-oncology. 2013;111:195–203. doi: 10.1007/s11060-012-1001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38(4):229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 19.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27(14):2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zill N, Peterson J. Behavior Problems Index. Washingston: Child Trends, Inc; 1986. [Google Scholar]
- 21.Achenbach TM. Manual for the Child Behavior Checklist/418 and 1991 Profile. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- 22.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166(1 Pt 2):141–157. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 23.Ware JEJ. Version 2 of the SF-36 Health Survey. Lincoln: QualityMetric; 2003. [Google Scholar]
- 24.Vannatta K, Gerhardt C, Wells RJ, Noll RB. Intensity of CNS treatment for pediatric cancer: Prediction of social outcomes in survivors. Pediatric Blood Cancer. 2007;49:716–722. doi: 10.1002/pbc.21062. [DOI] [PubMed] [Google Scholar]
- 25.Moyer KH, Willard VW, Gross AM, et al. The impact of attention on social functioning in survivors of pediatric acute lymphoblastic leukemia and brain tumors. Pediatric blood & cancer. 2012;59:1290–1295. doi: 10.1002/pbc.24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WIllard VW, Allen TA, Hardy KK, Bonner MJ. Social functioning in survivors of pediatric brain tumors: Contribution of neurocognitive and social-cognitive skills. Children’s Health Care. 2017;46(2):181–195. [Google Scholar]
- 27.Masten AS, Morison P, Pellegrini DS. A revised class play method of peer assessment. Developmental Psychology. 1985;21:523–533. [Google Scholar]
- 28.Barrera M, Schulte F. A group social skills intervention program for survivors of childhood brain tumors. Journal of Pediatric Psychology. 2009;38(10):1108–1118. doi: 10.1093/jpepsy/jsp018. [DOI] [PubMed] [Google Scholar]
- 29.DieTrill M, Bromberg J, LaVally B, Portales LA, SanFeliz A, Patenaude AF. Development of social skills in boys with brain tumours: A group approach. Journal of Psychosocial Oncology. 1996;14:2826–2835. [Google Scholar]
- 30.Barakat LP, Hetzke JD, Foley B, Carey ME, Gyato K, Phillips PC. Evaluation of a Social-Skills Training Group Intervention With Children Treated for Brain Tumors: A Pilot Study. Journal of Pediatric Psychology. 2003;28:299–307. doi: 10.1093/jpepsy/jsg019. [DOI] [PubMed] [Google Scholar]
- 31.Askins MA, Sahler OJ, Sherman SA, et al. Report from a multi-institutional randomized clinical trial examining computer-assisted problem-solving skills training for English- and Spanish-speaking mothers of children with newly diagnosed cancer. J Pediatr Psychol. 2009;34(5):551–563. doi: 10.1093/jpepsy/jsn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conklin HM, Reddick WE, Ashford J, et al. Long-term efficacy of methylphenidate in enhancing attention regulation, social skills, and academic abilities of childhood cancer survivors. J Clin Oncol. 2010;28(29):4465–4472. doi: 10.1200/JCO.2010.28.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conklin HM, Ogg RJ, Ashford JM, et al. Computerized Cognitive Training for Amelioration of Cognitive Late Effects Among Childhood Cancer Survivors: A Randomized Controlled Trial. J Clin Oncol. 2015;33(33):3894–3902. doi: 10.1200/JCO.2015.61.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roelofs RL, Wingbermuhle E, Egger JIM, Kessels RPC. Social Cognitive Interventions in Neuropsychiatric Patients: A meta-analysis. Brain Impairment. 2017;18(1):138–173. [Google Scholar]
- 35.Ness KK, Morris EB, Nolan VG, et al. Physical performance limitations among adult survivors of childhood brain tumors. Cancer. 2010;116(12):3034–3044. doi: 10.1002/cncr.25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armstrong GT, Sklar CA, Hudson MM, Robison LL. Long-term health status among survivors of childhood cancer: does sex matter? J Clin Oncol. 2007;25(28):4477–4489. doi: 10.1200/JCO.2007.11.2003. [DOI] [PubMed] [Google Scholar]
- 37.Poggi G, Liscio M, Galbiati S, et al. Brain tumors in children and adolescents: Cognitive and psychological disorders at different ages. Psycho-oncology. 2005;14:386–395. doi: 10.1002/pon.855. [DOI] [PubMed] [Google Scholar]
- 38.Mabbott DJ, Spiegler BJ, Greenberg ML, Rutka JT, Hyder DJ, Bouffet E. Serial evaluation of academic and behavioral outcome after treatment with cranial radiation in childhood. Journal of Clinical Oncology. 2005;23:2256–2263. doi: 10.1200/JCO.2005.01.158. [DOI] [PubMed] [Google Scholar]
- 39.Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncology. 2004;5:399–408. doi: 10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- 40.Wilson CL, Gawade PL, Ness KK. Impairments that influence physical function among survivors of childhood cancer. Children (Basel) 2015;2(1):1–36. doi: 10.3390/children2010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schultz KAP, Ness KK, Whitton J, et al. Behavioral and social outcomes in adolescent survivors of childhood cancer: a report from the childhood cancer survivor study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:3649–3656. doi: 10.1200/JCO.2006.09.2486. [DOI] [PubMed] [Google Scholar]
- 42.Noll RB, Bukowski WM, Rogosch FA, LeRoy S, Kulkarni R. Social interactions between children with cancer and their peers: teacher ratings. J Pediatr Psychol. 1990;15(1):43–56. doi: 10.1093/jpepsy/15.1.43. [DOI] [PubMed] [Google Scholar]
- 43.Noll RB, Reiter-Purtill J, Vannatta K, Gerhardt CA, Short A. Peer relationships and emotional well-being of children with sickle cell disease: a controlled replication. Child neuropsychology : a journal on normal and abnormal development in childhood and adolescence. 2007;13:173–187. doi: 10.1080/09297040500473706. [DOI] [PubMed] [Google Scholar]
- 44.Zebrack BJ, Zevon MA, Turk N, et al. Psychological distress in long-term survivors of solid tumors diagnosed in childhood: a report from the childhood cancer survivor study. Pediatr Blood Cancer. 2007;49(1):47–51. doi: 10.1002/pbc.20914. [DOI] [PubMed] [Google Scholar]
- 45.Alderfer MA, Long KA, Lown EA, et al. Psychosocial adjustment of siblings of children with cancer: A systematic review. Psycho-oncology. 2010;19:789–805. doi: 10.1002/pon.1638. [DOI] [PubMed] [Google Scholar]
- 46.Campbell LK, Scaduto M, Sharp W, et al. A meta-analysis of the neurocognitive sequelae of treatment for childhood acute lymphocytic leukemia. Pediatr Blood Cancer. 2007;49(1):65–73. doi: 10.1002/pbc.20860. [DOI] [PubMed] [Google Scholar]
- 47.Iyer NS, Balsamo LM, Bracken MB, Kadan-Lottick NS. Chemotherapy-only treatment effects on long-term neurocognitive functioning in childhood ALL survivors: a review and meta-analysis. Blood. 2015;126(3):346–353. doi: 10.1182/blood-2015-02-627414. [DOI] [PubMed] [Google Scholar]
- 48.Butler RW, Sahler OJ, Askins MA, et al. Interventions to improve neuropsychological functioning in childhood cancer survivors. Dev Disabil Res Rev. 2008;14(3):251–258. doi: 10.1002/ddrr.33. [DOI] [PubMed] [Google Scholar]
- 49.Barrera M, Atenafu EG, Sung L, et al. A randomized control intervention trial to improve social skills and quality of life in pediatric brain tumor survivors. Psycho-oncology. 2017 doi: 10.1002/pon.4385. [DOI] [PubMed] [Google Scholar]
- 50.Devine KA, Bukowski WM, Sahler OJ, et al. Social Competence in Childhood Brain Tumor Survivors: Feasibility and Preliminary Outcomes of a Peer-Mediated Intervention. J Dev Behav Pediatr. 2016;37(6):475–482. doi: 10.1097/DBP.0000000000000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


