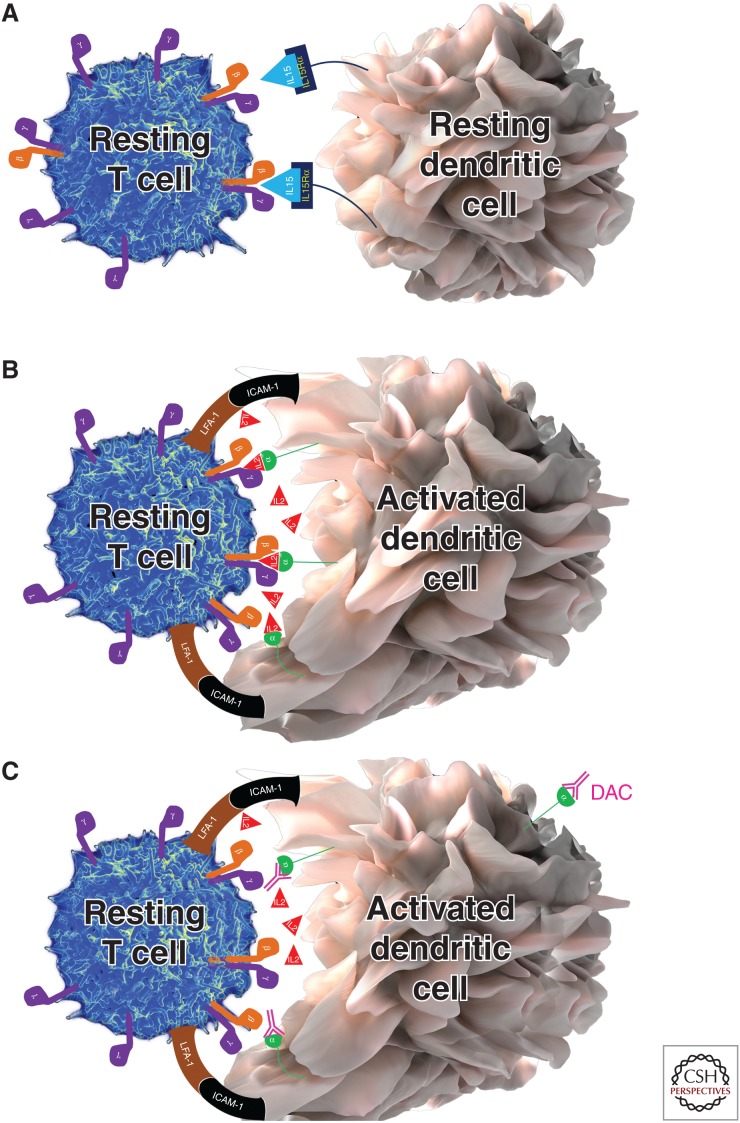
Figure 2.
Trans-presentation of interleukin (IL)-15 and IL-2. As a result of the very high affinity of the IL-15Rα chain for IL-15, the IL-15Rα chain can quickly capture and hold released IL-15 for long time periods. This IL-15Rα/IL-15 complex can then be readily trans-presented to intermediate affinity IL-2/IL-15 receptor, expressed on T cells or natural killer (NK) cells. In contrast, CD25 (IL-2Rα chain) only has low affinity for IL-2, so it is most unlikely that CD25 would be able to effectively capture small concentrations of IL-2 and create stable CD25/IL-2 complexes for trans-presentation, if IL-2 can readily diffuse to the environment. However, if IL-2 is released instead into the synaptic cleft, this is represented by an enclosed space between a peripheral supramolecular activation cluster formed by adhesion molecules such as lymphocyte function associated antigen 1 (LFA-1) and intercellular adhesion molecule 1 (ICAM-1), then diffusion is limited and high IL-2 concentrations can be achieved. Under those circumstances, when the T cell does not yet express CD25, CD25 is expressed on the surface of dendritic cells (DCs), which form stable immune synapses (IS) with antigen-specific T cells, and can effectively capture released IL-2 and trans-present it to the T cell. This cytokine signal is called signal 3. Signal 3 is delivered concomitantly with signal 1, provided by T-cell receptor (TCR) recognizing the peptide-MHC complex, and the costimulatory signal 2 (e.g., provided by interaction of CD28 with CD80 or CD86). All three signals seem necessary for efficient activation of human naïve T cells. When CD25 on the DC is blocked by daclizumab, then the primed T cell is unable to receive the IL-2-mediated signal 3, resulting in suboptimal stimulation of the T cell. Functional consequences are inhibition of antigen-specific T-cell activation, formation of antigen-specific effector, and memory T cells.