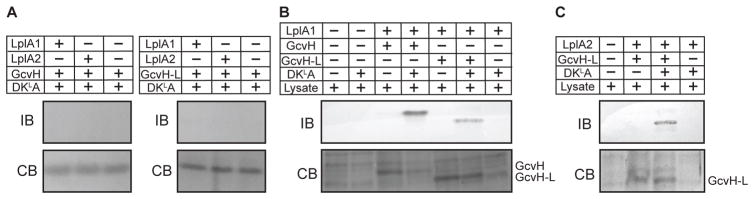
Figure 6. S. aureus crude lysates harbor lipoamidase activity that permits the use of DKLA as a source of lipoic acid for attachment by LplA1 and LplA2.
(A) LplA1 and LplA2 attachment of DKLA tripeptide-derived lipoic acid (2.4 mM) to GcvH and GcvH-L. Lipoylation was assessed by conducting immunoblots (IB) with rabbit α-lipoic acid antibody. A parallel 12% SDS-PAGE gels was stained with GelCode Blue (CB). (B) LplA1-dependent attachment of DKLA tripeptide-derived lipoic acid (2.4 mM) to GcvH, GcvH-L and (C) LplA2 attachment of DKLA tripeptide-derived lipoic acid (2.4 mM) to GcvH-L in the presence of S. aureus whole cell lysates derived from a ΔlipA ΔlipM ΔlipL ΔlplA1 ΔlplA2 mutant. Lipoylation was assessed by conducting immunoblots (IB) with rabbit α-lipoic acid antibody. A parallel 12% SDS-PAGE gel was stained with GelCode Blue (CB). The positions of GcvH and GcvH-L are indicated.