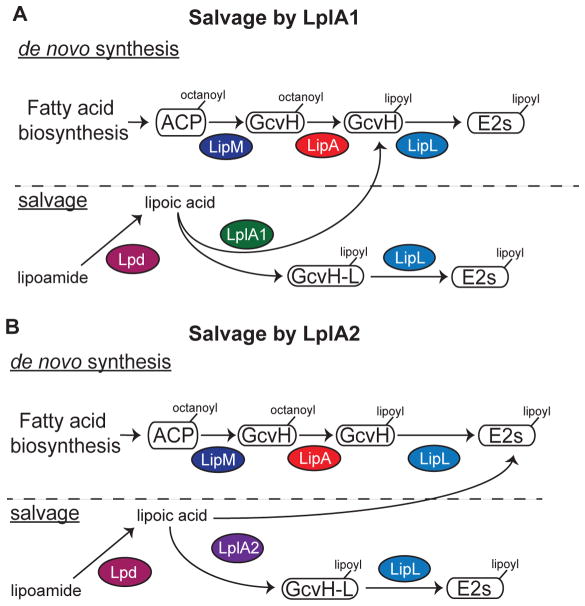
Figure 8. Model of lipoic acid salvage by LplA1 and LplA2.
(A–B) de novo biosynthesis of lipoic acid occurs via an octanoic acid precursor attached to acyl carrier protein (ACP). The octanoyl group is transferred to the H subunit of GcvH by the octanoyl transferase LipM. The octanoyl moiety is converted to lipoic acid by LipA and transferred to the lipoyl domains of E2-PDH, E2-OGDH, or E2-BCODH by the amidotransferase LipL. (A) LplA1 scavenges free lipoic acid and primarily attaches the cofactor to the H subunits of GcvH and GcvH-L followed by transfer to E2 subunits by LipL. (B) LplA2 also scavenges free lipoic acid, but instead attaches the cofactor to the H subunit of GcvH-L as well as the E2 subunits of PDH, OGDH, and BCODH. The activity of both LplA1 and LplA2 requires free lipoic acid. However, S. aureus harbors lipoamidase (Lpd) activity that can hydrolyze the amide bond of lipoamide, providing an additional source of free lipoic acid for use by these ligases.