Figure 4.
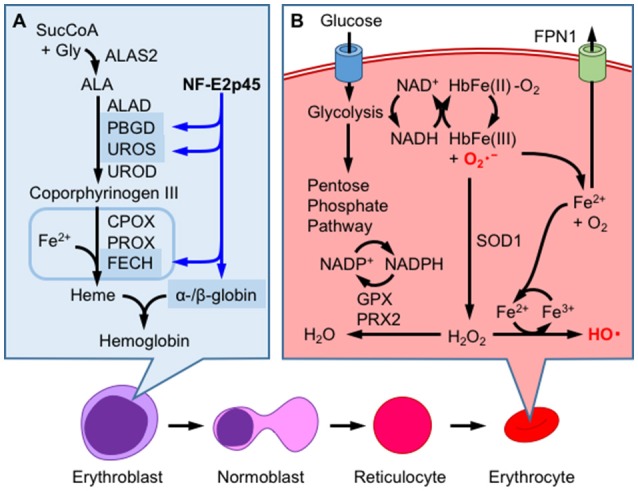
Hemoglobin production and redox homeostasis in erythrocytes. (A) NF-E2p45 participates in the transcriptional activation of α- and β-hemoglobin, as well as heme-synthetic enzymes, such as porphobilinogen deaminase (PBGD), uroporphyrinogen III synthase (UROS), and ferrochelatase (FECH), in erythroblasts. After enucleation, erythrocytes combat oxidative stress inducined by hemoglobin autoxidation through a mechanism that is not regulated by gene transcription. (B) Glucose metabolism supplies NADH, which is utilized for methemoglobin reduction. Superoxide anions () are eliminated by superoxide dismutase 1 (SOD1), which converts these ions into hydrogen peroxide (H2O2), or are converted into oxygen through heme degradation. Ferric iron reacts with by Haber-Weiss reaction to generate highly reactive hydroxyl radical (HO•) and oxygen molecule. H2O2 is further reduced to water by the consumption of NADPH by glutathione peroxidase (GPX) and peroxiredoxin 2 (PRX2); NADPH is produced by the pentose phosphate pathway. FPN1 exports cellular labile iron to prevent ROS production.
