Figure 3. Compatibility and orthogonality of the diazaborine chemistry to biological systems.
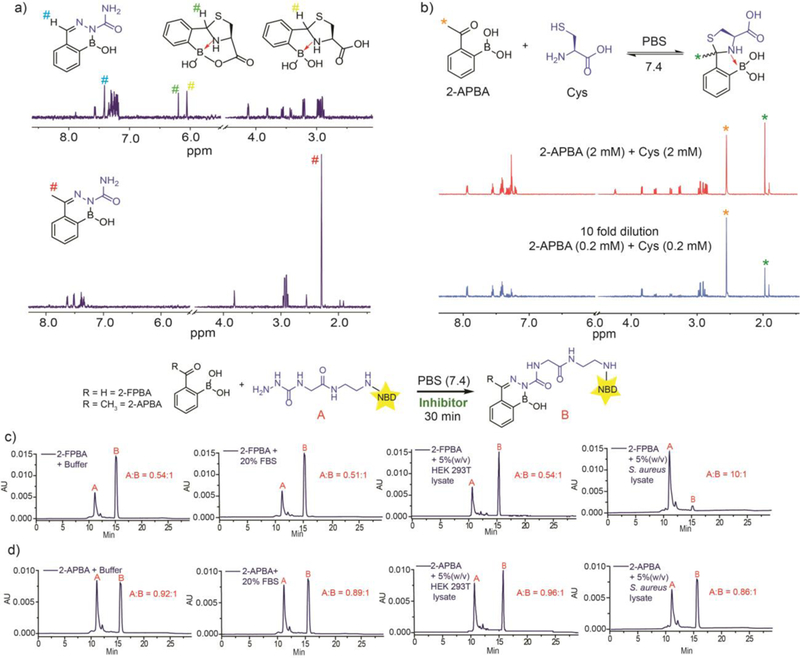
a) 1H-NMR spectra illustrating the weak affinity and rapid reversibility of the 2-APBA-cysteine conjugation. b) 1H-NMR analysis showing that the diazaborine formation of 2-FPBA, but not 2-APBA, is inhibited by the presence of free cysteine. All reactants were used at 0.2 mM in a pH 7.4 buffer. The reaction mixtures were incubated for 40 min before analysis. c) DAB1 and d) DAB2 formation in buffer alone and in presence of FBS (20%, v/v), HEK cell lysate (5 mg/mL), S. aureus cell lysate (5 mg/mL) respectively. For c) and d), the samples were prepared with 25 µM 2-FPBA or 2-APBA and 50 µM Scz-NBD in a pH 7.4 buffer. The LC traces were recorded after 30 min incubation. The relative peak areas of Scz-NBD over the diazaborine product are shown on the LC traces and used to estimate the conversion of the reactions.
