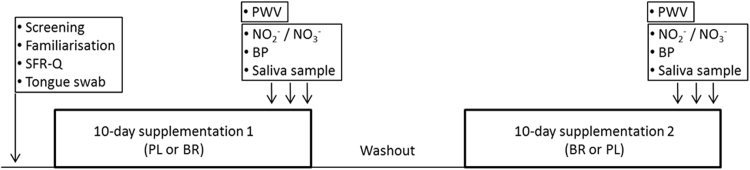
Fig. 1.
Participants underwent 10-day supplementation periods with nitrate (~ 12.4 mmol/d) and placebo in a balanced cross-over design. Screening, protocol familiarisation and Salivary Flow Rate Questionnaires (SFR-Q) were completed at baseline. Measurements of plasma nitrite (NO2-) and nitrate (NO3-) concentrations, blood pressure (BP) of the brachial artery, arterial stiffness as carotid-femoral pulse wave velocity (PWV), and the collection of saliva samples for microbiome analysis were undertaken on days 8, 9 and 10 of each supplementation period.