Figure 2 |. Reaction scheme and proposed mechanism for sp3 C–H arylation via a dual polyoxometalate HAT and nickel catalytic manifold.
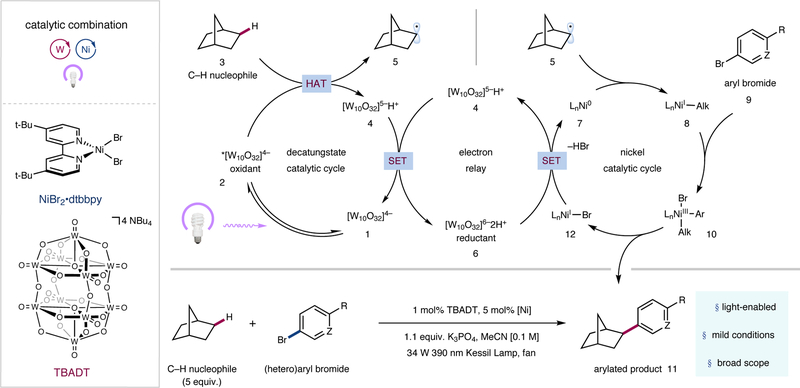
The catalytic cycle begins with photoexcitation of the decatungstate anion 1 to provide triplet excited state 2. Hydrogen atom transfer (HAT) from nucleophile 3 affords reduced photocatalyst 4 and open-shell species 5. Disproportionation of the reduced decatungstate species regenerates the active photocatalyst and affords the reducing hexa-anion 6. Ni0 species 7 subsequently captures alkyl radical 5, furnishing NiI-alkyl species 8. Oxidative addition into aryl electrophile 9 provides NiIII species 10, which undergoes reductive elimination to afford the product (11) and NiI–Br species 12. Single electron transfer (SET) between 6 and 12 regenerates 7 and 4, closing both catalytic cycles. dtbbpy, 4,4′-di-tert-butyl-2,2′-dipyridyl; TBADT, tetrabutylammonium decatungstate; Me, methyl; t-Bu, tert-butyl.
