Figure 3 |. Scope of the alkyl nucleophile coupling partner.
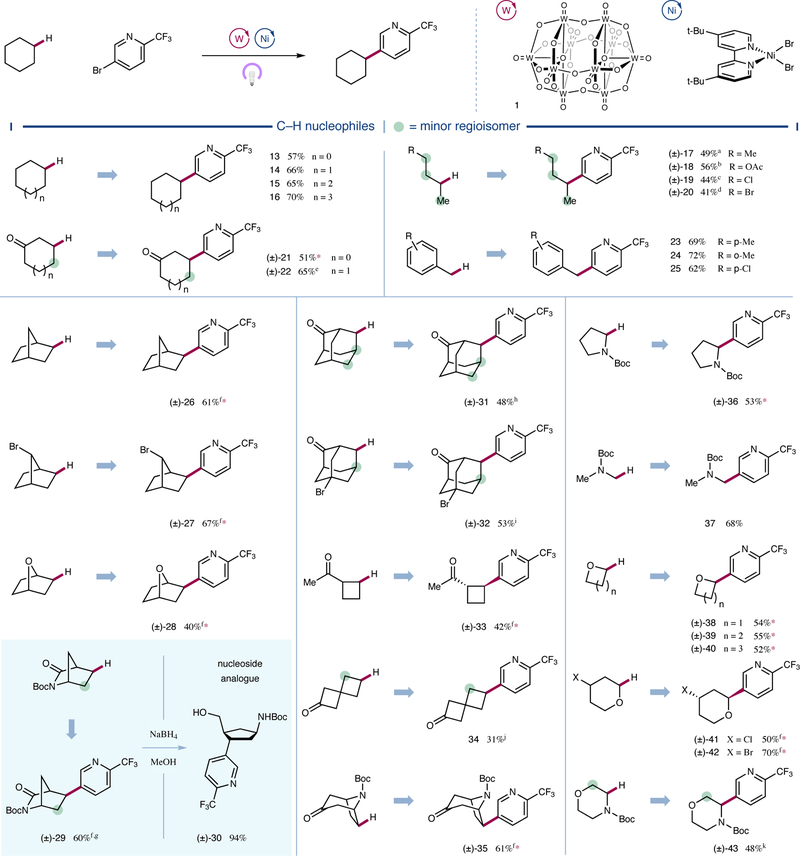
A broad range of C–H nucleophiles are selectively functionalized by this arylation protocol. Cyclic, acyclic, and bicyclic aliphatic systems are amenable substrates. Heteroatom and carbonyl substituents electronically influence regioselectivity, and alkyl halides remain intact. All yields are isolated yields. Conditions as in Figure 2. Green circles denote sites where significant amounts of other regioisomers are observed. See Supplementary Information for experimental details. Ac, acetyl; d.r., diastereomeric ratio; r.r., regioisomeric ratio. *>20:1 r.r.; a70% selectivity; b53% selectivity; c79% selectivity; d93% selectivity; e1.4:1 r.r.; f>20:1 d.r; g2.5:1 r.r.; h79% selectivity, 3:1 d.r. (major); i1.8:1 r.r., 5.4:1 d.r. (major); j8.8:1 r.r.; k3.4:1 r.r.
