Abstract
Purpose
Previous studies comparing extracorporeal membrane oxygenation (ECMO) modality for congenital diaphragmatic hernia (CDH) have not accounted for confounding by indication. We therefore hypothesized that using a propensity score (PS) approach to account for selection bias may identify outcome differences based on ECMO modality for infants with CDH.
Methods
We utilized ELSO Registry data (2000–2016). Patients with CDH were divided to either venoarterial (VA) or venovenous (VV) ECMO. Patients were matched by PS to control for non-random treatment assignment. Subgroup analyses were conducted based on timing of CDH repair relative to ECMO. Primary analysis was the “intent-to-treat” cohort based on the initial ECMO mode. Mortality was the primary outcome, and severe neurologic injury (SNI) was a secondary outcome.
Results
PS matching (3:1) identified 3,304 infants (VA=2,470, VV=834). In the main group, mortality was not different between VA and VV ECMO (OR=1.01, 95%CI:0.86–1.18) and there was no difference in SNI between VA and VV (OR=0.80; 95%CI:0.63–1.01). For the pre-ECMO CDH repair subgroup, 175 VA cases were matched to 70 VV. In these neonates, mortality was higher for VV compared to VA (OR=2.10, 95%CI:1.19–3.69), without any difference in SNI (OR=1.48; 95%CI:0.59–3.71). For the subgroup that did not have pre-ECMO CDH repair, 2,030 VA cases were matched to 683 VV cases. In this subgroup, VV was associated with 27% lower risk of SNI relative to VA (OR=0.73, 95%CI:0.56–0.95) without any difference in mortality (OR=0.94, 95%CI:0.79–1.11).
Conclusion
This study revalidates that ECMO mode does not significantly affect mortality or SNI in infants with CDH. In the subset of infants who require pre-ECMO CDH repair, VA favors survival. Whereas, in the subgroup of infants that did not have pre-ECMO CDH repair, VV favors lower rates of SNI. We conclude that neither mode appears consistently superior across all situations, and clinical judgement should remain a multifactorial decision.
Keywords: ECMO, CDH, venovenous, venoarterial, propensity score
1. INTRODUCTION
Congenital diaphragmatic hernia (CDH) is the leading indication for neonatal respiratory ECMO[1]. Two distinct cannulation options currently exist, venoarterial (VA) and venovenous (VV) ECMO. Both methods offer unique risks and benefits. VA ECMO is the most common cannulation method for CDH due to several theoretical factors: (1) Compared to other conditions that require neonatal ECMO, the physiologic consequences of pulmonary hypoplasia of CDH can be, or are perceived as, more severe for which VA ECMO is favored, (2) VA ECMO is less cumbersome to provide and manage at the bedside, and experience with VA ECMO is greater in most institutions, (3) starting with VA avoids the possibility of conversion from VV, and there may exist anatomic reasons where VV may not be an option[2, 3]. All previous research comparing the two modalities has not demonstrated a survival difference between VV and VA ECMO for CDH [4–6]. In 2015, APSA Outcomes committee’s systematic review on CDH stated that mode of ECMO modality has little bearing on CDH outcomes [7].
Previous body of work comparing VV and VA ECMO in infants with CDH includes previously noted limitations relating to the retrospective nature of these studies and the data elements that were available for risk stratification [8]. Although the data elements within the ELSO registry have not changed significantly, additional years of data have been collected since the last report on this subject. Furthermore, previous studies did not include statistical methodology to account for selection bias in selecting an ECMO mode of support. Thus, we undertook this study to address the efficacy of VA vs. VV using propensity score matched cohorts to minimize the influence of selection bias using a contemporary ELSO dataset. More specifically, our current study aimed to: 1) determine the efficacy of VA/VV with respect to the primary outcome, patient mortality, under an intention to treat (ITT) model; 2) examine whether treatment outcome differs in populations with and without pre-ECMO repair of diaphragmatic hernia; and 3) assess the extent to which inclusion/exclusion of neonates with VV to VA conversion affect treatment outcomes. Lastly, the study sought to compare VV to VA ECMO for patients with respect to severe neurologic injury (SNI) as a secondary outcome.
2. METHODS
2.1 Data Source and Cohort
This study was approved by the Children’s Hospital Orange County institutional review board (#150969). The ELSO Registry collects clinical information for adults and children with cardiorespiratory failure treated with ECMO. We queried the ELSO registry data for neonates whose primary diagnosis was CDH from 2000 to 2016. The study population consisted of neonates whose primary diagnosis was CDH. Every subject in the dataset was treated with ECMO. An exhaustive search through all secondary ICD-9 diagnosis codes was conducted to establish dichotomous variables to identify the presence of comorbidities. The first ECMO run was used for each neonate. We excluded patients with missing sex and ECMO mode (N=51 patients) and the final cohort included 4,580 neonates with CDH, including right sided, left sided or bilateral, all of whom were treated with ECMO.
2.2 Outcomes, Treatment Variables, and Analysis Cohorts
The main exposure/treatment variable was ECMO mode, VA and VV. Neonates who were treated with VA or VA bypass with retrograde venous drainage and those that were converted from VA to VV (VA-VV) were all considered to be VA[4]. Neonates treated with venovenous with a double lumen cannula (VVDL), VVDL with retrograde venous drainage (VVDL+V), and VV to VA conversion (VV-VA) were all considered VV[4]. The primary analysis cohort was the “intent-to-treat” cohort (ITT) defined based on the initial ECMO mode received. We note the distinction being made here that the “ITT” cohort should not be based on exclusion of neonates based on mode conversion as it would be unknown whether the ECMO mode would change for a neonate at the time of initiating ECMO, analogous to the assignment of treatment in a randomized controlled trial (RCT). Thus, appropriately, we explored in a post-hoc (secondary) analysis whether exclusion of infants with VV-VA conversion affected the results. This cohort is referred to as a “conditional cohort,” defined retrospectively conditioned on knowing VV-VA conversion status (which would not be known at the time of initial treatment assignment/determination). For the primary and secondary outcome variables, we further examined whether efficacy differed based on if the diaphragmatic hernia repair was performed prior ECMO (pre-ECMO repair), during ECMO (on-ECMO repair), or after liberation from ECMO (post-ECMO repair).
The primary outcome was patient mortality at discharge. The secondary outcome was severe neurologic injury (SNI), which was defined as a composite of acute neurological events (CNS hemorrhage, infarct, and/or intraventricular hemorrhage (IVH) grade 3&4) during ECMO represented in ELSO Registry[9]. Cerebral infarction or intracerebral hemorrhage (ICH) reported to the ELSO Registry are diagnosed using head ultrasonography or computerized tomography[10]. We excluded seizures, grade I, and grade II IVH from the definition of SNI[9, 11, 12]. Furthermore, we excluded brain death, which is also listed as a neurologic complication by ELSO, from the definition of SNI as this would lead to confounding with mortality. SNI therefore only measures severe neurologic insults during the same admission as ECMO.
2.3 Statistical Analysis
Propensity score (PS) matching[13] was performed to select the matched study cohorts with a feasible maximum of three VA’s per VV treatment case. A 3:1 match was feasible and afforded for maximal patients to be included in the study. The PS matching caliper was 0.15 times the standard deviation of the PS in the VV ECMO group. The caliper is a set threshold for matching the PS values between two patients in each of the treatment groups (i.e. PS for a patient on VV ECMO to a patient on VA ECMO). A smaller caliper threshold represents a more stringent matching criterion. PS’s were estimated based on a logistic regression model on the response variable as VV/VA treatment with pre-ECMO covariates: demographics variables including gender, birthweight (BW), race, gestational age (GA), post-gestational age, 5min Apgar, side of CDH, prenatal diagnosis of CDH, CDH repair prior to ECMO, hand-bagging and pre-ECMO arrest; blood gas/ventilator variables included pH, pCO2, and pO2, mean airway pressure (MAP), oxygenation index (OI); pre-ECMO rescue therapies included inotropes, bicarbonate/THAM, nitric oxide, surfactant, neuromuscular blockers, milrinone, sildenafil and steroids; comorbidity variables included critical congenital heart disease (CCHD), multiple congenital anomalies (MCA), chromosomal anomalies and perinatal infection. Missing values of BW, GA, pCO2, pO2 and OI were obtained using mean imputation (missing rate: 3.7–10.3%).
Continuous variables were reported as mean with standard deviation (SD) and compared using two-sample t-test and categorical variables were compared using chi-square test before and after PS matching. Univariate logistic regression models based on the matched sets were the primary analyses used to estimate odds ratio and 95% confidence interval (CI) within the matched set. Sensitivity analyses based on multivariate logistic regression adjusting for risk factors used in the matching yielded similar results and are not reported. Analyses were performed using SAS version 9.3 and R version 3.2.2.
3. RESULTS
3.1 Cohorts and Unmatched Baseline Characteristics
We identified 4,580 neonates with CDH who were treated with ECMO from 2000–2016 (partial latest data for 2016). The overall mortality was 2,391 (52.21%). There were 1,234 patients (26.94%) who were liberated from ECMO and did not survive to discharge. VA and VV ECMO were initially used in 82% and 18% of neonates, respectively (3743 in VA and 837 in VV). Of these 837 VV cases, 125 were later converted from VV to VA (15% of all VV cases and 3% of the entire cohort). Observed mortality rate in VV-VA conversion group was more than 10–15% higher than other groups (conversion: 63.2%, VA: 52.6% and VV: 48.0%). The prevalence of SNI and proportion of death or SNI in the conversion group were also the highest among all mode groups (Table 1). The percentage of SNI in VA, VV and conversion groups were 15.4%, 13.9% and 18.4%, respectively. There were 441 neonates in whom CDH repair was performed prior to being placed on ECMO, compared to 3790 who were not repaired prior to ECMO. Information on timing of CDH repair was missing for 349 infants. The overall rate of pre-ECMO CDH repair was 9.6% in the full cohort. The pre-ECMO repair rate in VV-VA conversion group was much higher than VA and VV groups (9.8% in VA, 6.9% in VV and 19.2% in conversion).
Table 1.
Frequency of mortality and SNI by mode of ECMO.
| Mode | N | Death | SNI |
|---|---|---|---|
| VA | 3743 | 1970 (52.63%) | 578 (15.44%) |
| VV | 712 | 342 (48.03%) | 99 (13.90%) |
| VV-VA | 125 | 79 (63.20%) | 23 (18.40%) |
| Total | 4580 | 2391 (52.21%) | 677 (14.78%) |
Multiple baseline characteristics including demographics, blood gas/ventilator, pre-ECMO rescue therapies and comorbidity variables were found to be significantly different between VA and VV in both ITT cohort and the cohort with exclusion of infants with VV-VA conversion before propensity score matching. In ITT cohort, neonates treated with VV ECMO were associated with heavier birthweight, more left side diaphragmatic hernia, less infants arrested before ECMO, higher pH, more frequent use of nitric oxide and neuromuscular blockers, less use of milrinone and sildenafil, and lower prevalence of CCHD relative to VA ECMO (Table 2). After the exclusion of VV-VA conversion, lower incidence of pre-ECMO CDH repair and higher rate of HFOV were noted within the VV group (data not shown). There was no difference in baseline patient characteristics between VV and VA groups after matching.
Table 2.
Demographics, Pre-ECMO blood gas/ventilator, pre-ECMO rescue therapies and comorbidity variables in neonates supported with VA or VV ECMO in ITT cohort before and after propensity score matching.
| Before Matching (N = 4,578) | After Matching (N = 3,304) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Category | VA (N = 3,743) N (Percent)/ Mean (SD) |
VV (N = 835) N (Percent)/ Mean (SD) |
Standardized Difference |
P | VA (N = 2,470) N (Percent)/ Mean (SD) |
VV (N = 834) N (Percent)/ Mean (SD) |
Standardized Difference |
P |
| Sex | Female | 1603 (42.83) | 343 (41.08) | −0.02 | 0.35 | 1013 (41.01) | 343 (41.13) | 0.002 | 0.95 |
| Male | 2140 (57.17) | 492 (58.92) | 1457 (58.99) | 491 (58.87) | |||||
| BW | 3.03 (0.50) | 3.12 (0.47) | 0.19 | <0.01 | 3.10 (0.49) | 3.12 (0.47) | 0.04 | 0.31 | |
| Race | Whites | 2270 (60.65) | 521 (62.40) | 0.06 | 0.48 | 1540 (62.35) | 520 (62.35) | 0.02 | 0.95 |
| Hispanics | 658 (17.58) | 135 (16.17) | 414 (16.76) | 135 (16.19) | |||||
| Blacks | 458 (12.24) | 109 (13.05) | 321 (13.00) | 109 (13.07) | |||||
| Others | 357 (9.54) | 70 (8.38) | 195 (7.89) | 70 (8.39) | |||||
| GA | 7.67 (6.94) | 7.66 (6.98) | −0.00 | 0.98 | 7.76 (7.15) | 7.66 (6.99) | −0.01 | 0.72 | |
| 5min Apgar | 6.23 (1.99) | 6.19 (1.91) | −0.01 | 0.63 | 6.22 (1.95) | 6.18 (1.91) | −0.01 | 0.68 | |
| Age (days) | 2.41 (3.96) | 2.26 (3.52) | −0.03 | 0.32 | 2.25 (3.63) | 2.26 (3.52) | 0.004 | 0.90 | |
| CDH Side | Left | 2696 (72.03) | 630 (75.45) | 0.13 | 0.001 | 1865 (75.51) | 630 (75.54) | 0.002 | 0.99 |
| Right | 829 (22.15) | 160 (19.16) | 475 (19.23) | 160 (19.18) | |||||
| Both | 108 (2.89) | 12 (1.44) | 36 (1.46) | 12 (1.44) | |||||
| Missing | 110 (2.94) | 33 (3.95) | 94 (3.81) | 32 (3.84) | |||||
| Prenatal diagnosis | 2506 (66.95) | 545 (65.27) | −0.03 | 0.35 | 1618 (65.51) | 545 (65.35) | −0.003 | 0.93 | |
| Pre-ECMO CDH repair | No | 3106 (82.98) | 684 (81.92) | 0.08 | 0.07 | 2050 (83.00) | 684 (82.01) | 0.02 | 0.78 |
| Yes | 368 (9.83) | 73 (8.74) | 209 (8.46) | 73 (8.75) | |||||
| Missing | 269 (7.19) | 78 (9.34) | 211 (8.54) | 77 (9.23) | |||||
| Hand bagging | No | 3463 (92.52) | 782 (93.65) | 0.04 | 0.52 | 2306 (93.36) | 781 (93.65) | 0.03 | 0.67 |
| Yes | 195 (5.21) | 37 (4.43) | 104 (4.21) | 37 (4.44) | |||||
| Missing | 85 (2.27) | 16 (1.92) | 60 (2.43) | 16 (1.92) | |||||
| Pre-ECLS Arrest | 327 (8.74) | 49 (5.87) | −0.11 | 0.006 | 136 (5.51) | 49 (5.88) | 0.01 | 0.68 | |
| pH | 7.17 (0.17) | 7.19 (0.16) | 0.12 | 0.002 | 7.19 (0.16) | 7.19 (0.16) | −0.001 | 0.81 | |
| pco2 | 68.94 (28.18) | 67.61 (25.45) | −0.04 | 0.20 | 66.83 (26.92) | 67.63 (25.45) | 0.03 | 0.45 | |
| po2 | 39.32 (30.13) | 38.30 (21.11) | −0.03 | 0.35 | 38.51 (25.26) | 38.28 (21.12) | −0.009 | 0.81 | |
| HFOV | 2707 (72.32) | 624 (74.73) | 0.05 | 0.15 | 1823 (73.81) | 623 (74.70) | 0.02 | 0.61 | |
| MAP | 16.50 (4.23) | 16.55 (4.33) | 0.01 | 0.78 | 16.52 (4.21) | 16.55 (4.34) | 0.006 | 0.87 | |
| Oxygenation index | 54.18 (33.64) | 53.33 (31.09) | −0.02 | 0.50 | 53.27 (32.13) | 53.36 (31.10) | 0.002 | 0.94 | |
| Inotropes | 3290 (87.90) | 739 (88.50) | 0.01 | 0.62 | 2190 (88.66) | 738 (88.49) | −0.0055 | 0.89 | |
| Bicarbonate/THAM | 1211 (32.35) | 285 (34.13) | 0.03 | 0.32 | 822 (33.28) | 284 (34.05) | 0.016 | 0.68 | |
| Nitric oxide | 3014 (80.52) | 708 (84.79) | 0.11 | 0.004 | 2068 (83.72) | 707 (84.77) | 0.02 | 0.47 | |
| Surfactant | 595 (15.90) | 145 (17.37) | 0.03 | 0.29 | 414 (16.76) | 144 (17.27) | 0.01 | 0.73 | |
| Neuromuscular blockers | 2119 (56.61) | 533 (63.83) | 0.14 | 0.001 | 1525 (61.74) | 532 (63.79) | 0.04 | 0.29 | |
| Milrinone | 341 (9.11) | 57 (6.83) | −0.08 | 0.03 | 166 (6.72) | 57 (6.83) | 0.004 | 0.90 | |
| Sildenafil | 53 (1.42) | 4 (0.48) | −0.09 | 0.02 | 15 (0.61) | 4 (0.48) | −0.01 | 0.67 | |
| Steroids | 260 (6.95) | 58 (6.95) | −0.00 | 0.99 | 175 (7.09) | 58 (6.95) | −0.005 | 0.89 | |
| CCHD | 155 (4.14) | 11 (1.32) | −0.17 | <0.01 | 44 (1.78) | 11 (1.32) | −0.03 | 0.36 | |
| MCA | 13 (0.35) | 2 (0.24) | −0.01 | 0.62 | 11 (0.45) | 2 (0.24) | −0.03 | 0.41 | |
| Chromosomal | 32 (0.85) | 6 (0.72) | −0.01 | 0.69 | 19 (0.77) | 6 (0.72) | −0.005 | 0.88 | |
| Perinatal infection | 85 (2.27) | 12 (1.44) | −0.06 | 0.13 | 41 (1.66) | 12 (1.44) | −0.01 | 0.66 | |
Results/conclusions based on p-values from nonparametric tests were the same.
BW-Birthweight
GA-Gestational Age
Age- Post gestational Age
pH, pCO2, pO2 worst recorded value 6h prior to ECMO
OI-Calculated; worst value 6h prior to ECMO
CCHD- Critical Congenital Heart Disease (ICD 9 Code 746.01/745/745.1/745.2/745.31/745.32/745.33/746.1/746.11/746.2/747.41/747.1/746.7)
3.2 Propensity Score Matched ITT Cohort
3.2.1 Primary Outcome: Mortality
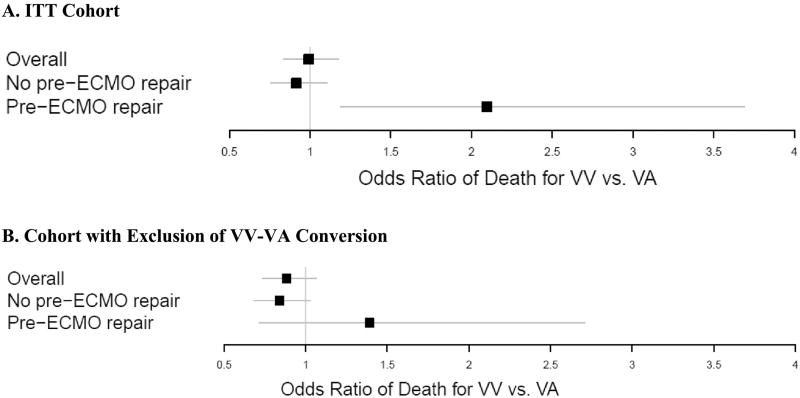
The propensity score matching identified 3,304 infants (VA = 2,470, VV = 834). Table 2 shows that there were no significant differences in demographics, blood gas/ventilator, pre-ECMO rescue therapies and comorbidity variables after PS matching. The odds of death were not significantly different between VA and VV ECMO treatment (OR = 1.01, 95% CI: 0.86–1.18, P = 0.95, Table 3A; Figure 2A).
Table 3.
Odds ratio of death between neonates treated with VV relative to VA ECMO in overall and subsets of with or without pre-ECMO CDH repair.
| A. ITT Cohort | ||||
|---|---|---|---|---|
| Parameter | Category | OR | 95% Confidence Interval |
P Value |
| ECMO mode | Overall | |||
| VA | 1.00 (Reference) | |||
| VV | 1.005 | (0.859–1.175) | 0.9541 | |
| No Pre-ECMO Repair | ||||
| VV | 0.937 | (0.788–1.114) | 0.4616 | |
| Pre-ECMO Repair | ||||
| VV | 2.096 | (1.192–3.687) | 0.0102 | |
| B. Cohort with Exclusion of VV-VA Conversion | ||||
|---|---|---|---|---|
| Parameter | Category | OR | 95% Confidence Interval |
P Value |
| ECMO mode | Overall | |||
| VA | 1.00 (Reference) | |||
| VV | 0.914 | (0.771–1.084) | 0.3026 | |
| No Pre-ECMO Repair | ||||
| VV | 0.857 | (0.711–1.033) | 0.1047 | |
| Pre-ECMO Repair | ||||
| VV | 1.391 | (0.714–2.711) | 0.3319 | |
Figure 2. Odds ratio of death between neonates treated with VA relative to VV ECMO in (A.) IIT cohort and (B.) the cohort with exclusion of VV-VA conversion.
A. ITT Cohort
B. Cohort with Exclusion of VV-VA Conversion
We next examined whether the finding of no treatment difference extends to the neonates who had pre-ECMO CDH repair compared to all others where CDH repair was not performed prior to ECMO. After matching, a total of 2,713 neonates were identified who did not have pre-ECMO CDH repair, of which 2,030 VA cases were matched to 683 VV cases. A plot of the standardized differences, showed no substantive difference in covariates between ECMO modes in the subpopulation (Figure 1A). Among those who did not have pre-ECMO repair, there was no significant difference in mortality between VA and VV with OR = 0.94 (95% CI: 0.79–1.11, P = 0.462, Table 3A).
Figure 1.
Depicts absolute standardized differences before (circles) and after matching (triangles). (Upper panels)- Includes intention-to-treat group (VV-VA conversion) as full cohort. (Lower Panels)- Excludes intention-to-treat group (VV-VA conversion). (A)- Matched VV and VA pairs among infants with no pre-ECMO CDH repair (Left), and with pre-ECMO CDH repair (Right). (B)- Depicts exclusion of VV-VA conversion (ITT group) from matched pairs. The threshold of 0.2 is the Cohen’s effect size index.
3.2.2 Secondary Outcome: SNI
We evaluated for potential differences in the secondary outcome, SNI, within the PS-matched groups. There was no difference in SNI between VA and VV ECMO (OR = 0.80; 95% CI: 0.63–1.01; P = 0.062, Table 4A) among the main PS matched group. For the subgroup of infants that did not have pre-ECMO CDH repair, VV was associated with 27% lower risk of SNI relative to VA (OR = 0.73; 95% CI: 0.56–0.95; P = 0.021).
Table 4.
Odds ratio of SNI between neonates treated with VV relative to VA ECMO in overall and subsets of with or without pre-ECMO CDH repair.
| A. ITT Cohort | ||||
|---|---|---|---|---|
| Parameter | Category | OR | 95% Confidence Interval |
P Value |
| ECMO mode | Overall | |||
| VA | 1.00 (Reference) | |||
| VV | 0.797 | (0.628–1.011) | 0.0620 | |
| No Pre-ECMO Repair | ||||
| VV | 0.733 | (0.564–0.954) | 0.0207 | |
| Pre-ECMO Repair | ||||
| VV | 1.484 | (0.593–3.711) | 0.3988 | |
| B. Cohort with Exclusion of VV-VA Conversion | ||||
|---|---|---|---|---|
| Parameter | Category | OR | 95% Confidence Interval |
P Value |
| ECMO mode | Overall | |||
| VA | 1.00 (Reference) | |||
| VV | 0.759 | (0.58–0.993) | 0.0441 | |
| No Pre-ECMO Repair | ||||
| VV | 0.694 | (0.521–0.925) | 0.0128 | |
| Pre-ECMO Repair | ||||
| VV | 0.159 | (0.02–1.234) | 0.0785 | |
3.3 Post-Hoc Analyses: Timing of CDH Repair
We further evaluated the cohort with pre-ECMO CDH repair, where 175 VA cases were matched to 70 VV. Among those with pre-ECMO repair, the odds of death for VV group was more than doubled compared to VA treatment (OR = 2.10, 95% CI: 1.19–3.69; P= 0.010). There was no difference in SNI within the cohort that underwent pre-ECMO CDH repair (OR = 1.48; 95% CI: 0.59–3.71; P = 0.399).
Subgroups by (a) on-ECMO repair (N=1290), (b) post-ECMO repair (N=928), and (c) no-repair (N=437) were also examined. (We note ELSO data does not provide reason for no-repair, such as failure to wean from ECMO.) With respect to our primary outcome, mortality, there were not differences in mortality within subgroups between VV and VA ECMO (all P > 0.25, OR = 0.990, 0.791, and 0.680 for subgroups a, b, and c, respectively), consistent with our original finding in the overall no pre-ECMO repair cohort; OR=0.937, P=0.4616).
With respect to our secondary outcome, SNI, overall OR estimates were also consistent with the original results for the overall no pre-ECMO repair cohort; however, the 27% lowered odds of SNI in the VV group for the overall no pre-ECMO repair cohort was driven by the no-repair subgroup (OR=0.415; 95% CI: 0.223-0.223–0.769; P=0.0052).
3.4 Post-Hoc Analyses with Exclusion of VV-VA Conversion
3.4.1 Mortality
After the exclusion of the VV-VA conversion group, the matching lead to 2,821 infants (VA = 2,113, VV = 708) in the main group. There were no significant differences in any patient covariates after matching (data not shown). Similar to the ITT analysis (where VV-VA conversion was included in the VV cohort for all analyses), there was no difference between VA and VV ECMO treatment on mortality (OR = 0.91, 95% CI: 0.77–1.08; P = 0.303, Table 3B; Figure 2B) among all infants with conversion excluded, although estimated OR of death was incrementally lower (OR = 0.91 vs. 1.01).
A total of 2,339 infants were matched that did not have pre-ECMO CDH repair (N = 1,748 VA, N = 591 VV). The plot of standardized difference is provided in Figure 1B. Among infants without pre-ECMO repair, VV and VA cohorts were not significantly different in mortality (OR = 0.86, 95% CI: 0.71–1.03; P = 0.105, Table 3B). Note that the direction of the OR is same as ITT results. However, different from the ITT results, mortality risk between VV and VA were not different among the matched cohort of 180 infants (133 VA; 37 VV) that underwent pre-ECMO CDH repair (OR = 1.39; 95% CI: 0.71–2.71; P = 0.332). This demonstrates that the exclusion of VV-VA conversion group accounts for the difference in mortality seen amongst those infants that underwent CDH repair and then required treatment with ECMO.
3.4.2 SNI
In the cohort of patients that excluded VV-VA conversion, VV was associated with a lower risk of SNI (OR = 0.76; 95% CI: 0.58–0.99; P = 0.044, Table 4B). Similar to the ITT analysis, infants treated with VV were associated with a lower risk of SNI relative to VA among infants that were not in the pre-ECMO CDH repair group (OR = 0.69; 95% CI: 0.52–0.93; P = 0.013). There was no difference in SNI within the cohort that had pre-ECMO CDH repair (OR = 0.16; 95% CI: 0.02–1.23; P = 0.079).
3.5 Sensitivity Analyses
3.5.1 ECMO Duration
A sensitivity (post hoc) analysis was conducted that included ECMO duration in the PS matching so that there would be no difference in ECMO duration between VA and VV groups. The mean (SD) of duration (weeks) of ECMO support before and after PS matching in VA vs. VV was 1.67 (1.06) vs. 1.72 (1.12) and 1.71 (1.06) vs. 1.72 (1.12) for unmatched and matched groups, respectively. In the analysis accounting for ECMO duration , there were no differences in the results compared to our main results. Specifically, there was no difference in mortality (OR = 0.970; 95% CI: 0.829–1.135; P = 0.7027) in the overall cohort ; for SNI, the results were also similar with lower risk of SNI among those who did not have pre-ECMO CDH repair (OR = 0.797; 95% CI: 0.565–0.957; P = 0.0223).
3.5.2 Years of ECMO: 2006–2016
Our main analysis included data from the years 2000–2016. We examined a more contemporary cohort based on the last 10 years of data as a sensitivity analysis. Similar to the original cohort, there was no difference in mortality (OR=0.957; 95% CI: 0.775–1.182; P=0.686). There was no difference in mortality between VV and VA ECMO for infants between 2006 and 2016 that underwent pre-ECMO repair (OR=1.558; 95 % CI: 0.679–3.357; P=0.295). However, the estimated risk of mortality was notably higher for this group overall, similar to the results of the original cohort (OR = 2.10, 95% CI: 1.19–3.69; P= 0.010). There was no difference in mortality for the cohort that did not undergo pre-ECMO repair (OR=0.976; 95% CI: 0.774–1.231; P=0.836). We note that the sensitivity analysis result for the cohort with pre-ECMO repair should be interpreted with caution given the reduction in sample size (N=108 neonates with pre-ECMO repair, a reduction of 38%).
Sensitivity analyses for the secondary outcome, SNI, also showed no difference in risk of SNI between VV and VA in the overall cohort (as well as the for cohort with pre-ECMO repair), consistent with analyses based on all years. However, for the cohort that did not undergo pre-ECMO repair risk of SNI estimate for VV relative to VA was similar (OR=0.875 vs. OR=0.733 for 10 years versus all years), although not statistically significant (95% CI: 0.623–1.230).
3.5.3. Exclusion of Pre-ECLS Arrest
Sensitivity analyses that excluded patients who arrested prior to ECMO, showed the results for the primary outcome, mortality, were essentially unchanged in all cohorts. The results were also similar for the secondary outcome, SNI, in the overall cohort and the cohort without pre-ECMO repair. For the cohort without pre-ECMO repair, the risk of SNI for VV vs. VA was incrementally, but not substantially, changed (OR=0.769; 95% CI: 0.584–1.012; P=0.0620).
4. DISCUSSION
Although a randomized controlled trial (RCT) would be the gold standard for identifying a clinical advantage of a specific ECMO modality, when applied to infants with CDH, the incidence, resources, time, and collaborative effort required limit the feasibility of this approach. This is further complicated by the heterogeneity in ECMO utilization rates and variation in treatment algorithms across centers making it near impossible to perform a clinical trial of this nature[14]. Propensity score matching is a feasible alternative for a retrospective observational study to minimize bias in the assessment of treatment efficacy, which is ideally achieved with a RCT study design. In this current study, the propensity score was developed from the data elements that were present within the ELSO Registry that identified choice of treatment of ECMO modality as an outcome. The main analysis matched 2,470 VA cases to 834 VV cases (3:1) based on the PS score. The differences seen in multiple baseline characteristics and pre-ECMO parameters between VA and VV groups were accounted for after PS matching. In the matched groups, the odds of death were not different between VA and VV ECMO. This overall result is in agreement with what was previously reported [4, 6, 15].
If VV ECMO is deemed insufficient to meet the hemodynamic needs of the patient, the only option is conversion to VA ECMO. Given that VV has a failure rate, we evaluated the VV-VA conversion rate and the specific mortality in this group. The VV conversion rate was 15% in this study and as a subgroup they had the greatest mortality rate, 10–15% higher compared to VA or VV cases that were not within the conversion group. In the main analysis, we included the VV-VA conversion group within the VV cohort, as the initial treatment intent in those infants was VV [16]. This therefore accounts for intention to treat bias in the main analysis. When we separately compared VV to VA ECMO after excluding the VV-VA conversion group from VV cases, there still was no statistically significant difference between the two groups within the PS matched groups. Clearly, the cases of VV that failed are playing a part in inflating the mortality rate within the VV cohort, however, there is still no difference in mortality at discharge—the primary outcome of the study—when we compare VV and VA where there were no converted cases. Proponents of VV may argue that those infants that failed VV may have failed VA had they been placed on VA initially, however there are no good means to test that hypothesis as the sample size is rather small. We also don’t know why infants died on VV without being converted to VA, and it is possible that some may have survived with conversion. These are tough situations and clinical clarity may not exist, yet the comparisons above demonstrate a statistically non-significant effect of VV-VA conversion group to overall survival.
Given the susceptibility for neurologic complications during ECMO [4, 10], we studied the odds of severe neurological complications attributable to ECMO. ELSO Registry does not contain long term neurodevelopmental outcomes, but other investigators have linked ECMO to developmental delay in the CDH population [10, 17, 18] as well as grade III/IV IVH to measurable delays in neonatal population [19]. Therefore, short of a true neurodevelopmental outcome that is measurable, we evaluated the odds of having SNI as the most significant adverse outcome that could be evaluated from the ELSO Registry. Within the PS-matched groups, there was no difference in the odds of having SNI between VV and VA ECMO. These results are different than previously reported [4, 10], but the secondary outcome we are measuring, as defined by SNI, is specifically focused on severe neurologic complications.
In order to understand the contribution of the VV-VA conversion group on described primary and secondary outcomes, we conducted an ITT analysis. The group of infants who were converted, had the highest mortality rate within the entire cohort, and these significantly increase the mortality rate for the VV cohort, making it indistinguishable from the VA group. Although, odds of mortality was lower, the exclusion of the conversion group did not lead to a mortality difference or SNI between the matched VV and VA cohorts. Overall, these results suggest clinical equipoise once again in that ECMO modality does not affect mortality or SNI in infants with CDH.
Whether there is a repair and the timing of repair of the diaphragmatic hernia is an integral part of the treatment plan that is inherently complicated by timing of being placed or liberated from ECMO [8, 20, 21]. Repair could be done prior to ECMO, during or after ECMO. Overall the optimal timing of CDH repair is not known and is variable depending on institutional preferences/beliefs [22]. When repair is performed during or after ECMO, this is often the preference of the treatment team. However, when ECMO is required following CDH repair, it may be that patient decompensated either as a result of the repair or despite the repair and options were limited requiring ECMO as salvage. We therefore performed a post-hoc analysis on the subgroup of infants who underwent CDH repair prior to ECMO. This subgroup was significantly smaller than the initial main comparison, with 441 out of 4580. Neonates treated with VV were associated with a two-fold increase in the odds of mortality compared to VA group among pre-ECMO repair infants in the ITT analysis, while the ECMO mode effect disappeared when we excluded the conversion. Furthermore, there was some evidence of significant difference in risk of SNI between VA and VV in both ITT and with conversion excluded analysis, meanwhile VV was associated with a lower risk of SNI compared to VA among infants without pre-ECMO repair, which matches previous observations that VA is associated overall with greater rate of neurologic complications[4].
There were multiple limitations to our study. We were unable to account or correct for potential data entry errors. Given the retrospective nature of the study, we were unable to account or correct for potential data entry errors. The error rate of ELSO Registry is believed to be near 1%[23]. Although the PS matching has strengths in terms of minimizing selection bias, it is limited by the available data and cannot account for unmeasured confounders (which is a unique advantage of RCTs). Another limitation of all ELSO based research is the selection bias of the Registry, in that every infant reported was subjected to ECMO, which in this case is not relevant as we are comparing outcomes related to mode of ECMO cannulation as both treatments (VV and VA) happen on ECMO. Timing (i.e. early or late) of conversion from VV to VA is not identified in the ELSO dataset, nor was reason for conversion (i.e. cannula issues, hemodynamic problems, or other reasons for VV failure). Had timing/reason for conversion from VV to VA been available, some of the conversions may have been treated differently in study design. Unfortunately, we were unable to account for center effect in is study, as ELSO does not, or no longer, releases center information for research. However, in our previous study comparing mode of ECMO[4], we were able to account for ECMO-center as a random effect, and it did not change the model estimates significantly. Risk stratification based on prenatal measurements and anatomic operative details have prognostic value for these infants, which we were not able to address because ELSO doesn’t collect that data. Given the significant variation in the measurement techniques for lung-head-ratio and that most centers do not report these in an observed to expected ratio at different gestational ages, it is very difficult to incorporate such measurements from multiple institutions that provide data to ELSO.
In summary, we re-tested the hypothesis that ECMO modality affects survival among infants with CDH with statistical methodology that attempts to mimic an RCT. Overall, our study showed that after PS matching, there is no significant difference in mortality based on the mode of ECMO among infants with CDH. Furthermore, mode of ECMO does not have an effect on SNI, i.e. acute severe neurological events during ECMO. Nevertheless, in infants who required ECMO after CDH repair, VA ECMO was associated with lower odds of mortality but greater odds of SNI. We conclude that mode of ECMO does not have a measurable effect on mortality in infants with CDH, therefore, ECMO providers should base their decisions on center experience and anatomic restraints in choosing between VV and VA ECMO. Lastly, a strong consideration should be given to preferring VA cannulation in those infants who require ECMO after CDH repair.
Acknowledgments
This work was supported by CHOC/Pediatric Subspecialty Faculty (PSF) Tithe Award and by grant UL1 TR001414 from the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through the Biostatistics, Epidemiology and Research Design Unit. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barbaro RP, Paden ML, Guner YS, Raman L, Ryerson LM, Alexander P, et al. Pediatric Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J. 2017 doi: 10.1097/MAT.0000000000000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frenckner B, Palmer K, Linden V. Neonates with congenital diaphragmatic hernia have smaller neck veins than other neonates-An alternative route for ECMO cannulation. J Pediatr Surg. 2002;37(6):906–8. doi: 10.1053/jpsu.2002.32908. [DOI] [PubMed] [Google Scholar]
- 3.Cassady CI, Mehollin-Ray AR, Olutoye OO, Cass DL. Jugular vein hypoplasia can preclude extracorporeal membrane oxygenation cannulation in the neonate with congenital diaphragmatic hernia: potential identification of the neonate at risk by fetal magnetic resonance imaging. Fetal Diagn Ther. 2011;30(3):225–8. doi: 10.1159/000330783. [DOI] [PubMed] [Google Scholar]
- 4.Guner YS, Khemani RG, Qureshi FG, Wee CP, Austin MT, Dorey F, et al. Outcome analysis of neonates with congenital diaphragmatic hernia treated with venovenous vs venoarterial extracorporeal membrane oxygenation. J Pediatr Surg. 2009;44(9):1691–701. doi: 10.1016/j.jpedsurg.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Heiss KF, Clark RH, Cornish JD, Stovroff M, Ricketts R, Kesser K, et al. Preferential use of venovenous extracorporeal membrane oxygenation for congenital diaphragmatic hernia. J Pediatr Surg. 1995;30(3):416–9. doi: 10.1016/0022-3468(95)90045-4. [DOI] [PubMed] [Google Scholar]
- 6.Kugelman A, Gangitano E, Pincros J, Tantivit P, Taschuk R, Durand M. Venovenous versus venoarterial extracorporeal membrane oxygenation in congenital diaphragmatic hernia. J Pediatr Surg. 2003;38(8):1131–6. doi: 10.1016/s0022-3468(03)00256-2. [DOI] [PubMed] [Google Scholar]
- 7.Puligandla PS, Grabowski J, Austin M, Hedrick H, Renaud E, Arnold M, et al. Management of congenital diaphragmatic hernia: A systematic review from the APSA outcomes and evidence based practice committee. J Pediatr Surg. 2015;50(11):1958–70. doi: 10.1016/j.jpedsurg.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Kays DW. ECMO in CDH: Is there a role? Semin Pediatr Surg. 2017;26(3):166–70. doi: 10.1053/j.sempedsurg.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Harting MT, Davis CF, Lally KP. Congenital Diaphragmatic Hernia and ECMO. Extracorporeal Life Support The ELSO Red Book. (5) 2017:133–50. [Google Scholar]
- 10.Polito A, Barrett CS, Wypij D, Rycus PT, Netto R, Cogo PE, et al. Neurologic complications in neonates supported with extracorporeal membrane oxygenation. An analysis of ELSO registry data. Intensive Care Med. 2013;39(9):1594–601. doi: 10.1007/s00134-013-2985-x. [DOI] [PubMed] [Google Scholar]
- 11.Clyman RI, Saha S, Jobe A, Oh W. Indomethacin prophylaxis for preterm infants: the impact of 2 multicentered randomized controlled trials on clinical practice. J Pediatr. 2007;150(1):46–50 e2. doi: 10.1016/j.jpeds.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Courtney SE, Durand DJ, Asselin JM, Hudak ML, Aschner JL, Shoemaker CT, et al. Highfrequency oscillatory ventilation versus conventional mechanical ventilation for very-low-birthweight infants. N Engl J Med. 2002;347(9):643–52. doi: 10.1056/NEJMoa012750. [DOI] [PubMed] [Google Scholar]
- 13.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Weems MF, Jancelewicz Tim, Sandhu Hitesh S. Congenital Diaphragmatic Hernia: Maximizing Survival. NeoReviews. 2016;17(12) [Google Scholar]
- 15.Dimmitt RA, Moss RL, Rhine WD, Benitz WE, Henry MC, Vanmeurs KP. Venoarterial versus venovenous extracorporeal membrane oxygenation in congenital diaphragmatic hernia: the Extracorporeal Life Support Organization Registry, 1990–1999. J Pediatr Surg. 2001;36(8):1199–204. doi: 10.1053/jpsu.2001.25762. [DOI] [PubMed] [Google Scholar]
- 16.Gupta SK. Intention-to-treat concept: A review. Perspect Clin Res. 2011;2(3):109–12. doi: 10.4103/2229-3485.83221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta A, Ibsen LM. Neurologic complications and neurodevelopmental outcome with extracorporeal life support. World J Crit Care Med. 2013;2(4):40–7. doi: 10.5492/wjccm.v2.i4.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman S, Chen C, Chapman JS, Jeruss S, Terrin N, Tighiouart H, et al. Neurodevelopmental outcomes of congenital diaphragmatic hernia survivors followed in a multidisciplinary clinic at ages 1 and 3. J Pediatr Surg. 2008;43(6):1035–43. doi: 10.1016/j.jpedsurg.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 19.Bolisetty S, Dhawan A, Abdel-Latif M, Bajuk B, Stack J, Lui K, et al. Intraventricular hemorrhage and neurodevelopmental outcomes in extreme preterm infants. Pediatrics. 2014;133(1):55–62. doi: 10.1542/peds.2013-0372. [DOI] [PubMed] [Google Scholar]
- 20.Partridge EA, Peranteau WH, Rintoul NE, Herkert LM, Flake AW, Adzick NS, et al. Timing of repair of congenital diaphragmatic hernia in patients supported by extracorporeal membrane oxygenation (ECMO) J Pediatr Surg. 2015;50(2):260–2. doi: 10.1016/j.jpedsurg.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Austin MT, Lovvorn HN, 3rd, Feurer ID, Pietsch J, Earl TM, Bartilson R, et al. Congenital diaphragmatic hernia repair on extracorporeal life support: a decade of lessons learned. Am Surg. 2004;70(5):389–95. discussion 95. [PubMed] [Google Scholar]
- 22.Harting MT, Lally KP. The Congenital Diaphragmatic Hernia Study Group registry update. Semin Fetal Neonatal Med. 2014;19(6):370–5. doi: 10.1016/j.siny.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Dalton HJ, Butt WW. Extracorporeal life support: an update of Rogers' Textbook of Pediatric Intensive Care. Pediatr Crit Care Med. 2012;13(4):461–71. doi: 10.1097/PCC.0b013e318253ca17. [DOI] [PubMed] [Google Scholar]