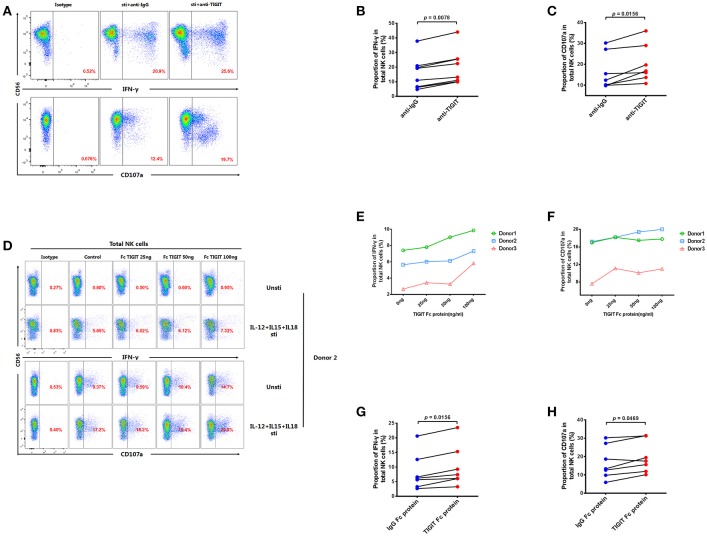
Figure 5.
Blocking TIGIT can restore the function of NK cells in HIV-infected individuals. (A) A representative flow cytometry plot showing the proportion of IFN-γ producing NK cells after treatment with 5 μg/mL anti-human TIGIT antibodies or purified mouse IgG (as a negative control) from HIV-infected individuals. The expression of IFN-γ was gated according to an isotype control. (B) Paired comparisons of the proportions of IFN-γ producing NK cells after treatment with 5 μg/mL anti-human TIGIT antibodies or purified mouse IgG (as a negative control) from HIV-infected individuals (n = 8). (C) Paired comparisons of the proportion of CD107a expressed by NK cells after treatment with 5 μg/mL anti-human TIGIT antibodies or purified mouse IgG (as a negative control) from HIV-infected individuals (n = 7). (D) A representative flow cytometry plot demonstrating the effects of different doses of rTIGIT (0 (medium only), 20, 50, and 100 ng/mL) on IFN-γ and CD107a production by NK cells. (E) Proportion of IFN-γ+ NK cells from HIV-infected individuals after treatment with different concentrations of rTIGIT (0, 20, 50, and 100 ng/mL; n = 3). (F) Proportion of CD107a+ NK cells from HIV-infected individuals after treatment with different concentrations of rTIGIT (0, 20, 50, and 100 ng/mL; n = 3). (G) Paired comparisons of the proportion of IFN-γ producing NK cells after treatment with 50 ng/mL rTIGIT or rIgG from HIV-infected individuals (n = 7). (H) Paired comparisons of the proportion of CD107a-expressing NK cells after treatment with 50 ng/mL rTIGIT or rIgG from HIV-infected individuals (n = 7). Wilcoxon matched-pairs signed-rank tests were used for comparisons between paired groups. p < 0.05 was considered significant.