Figure 1.
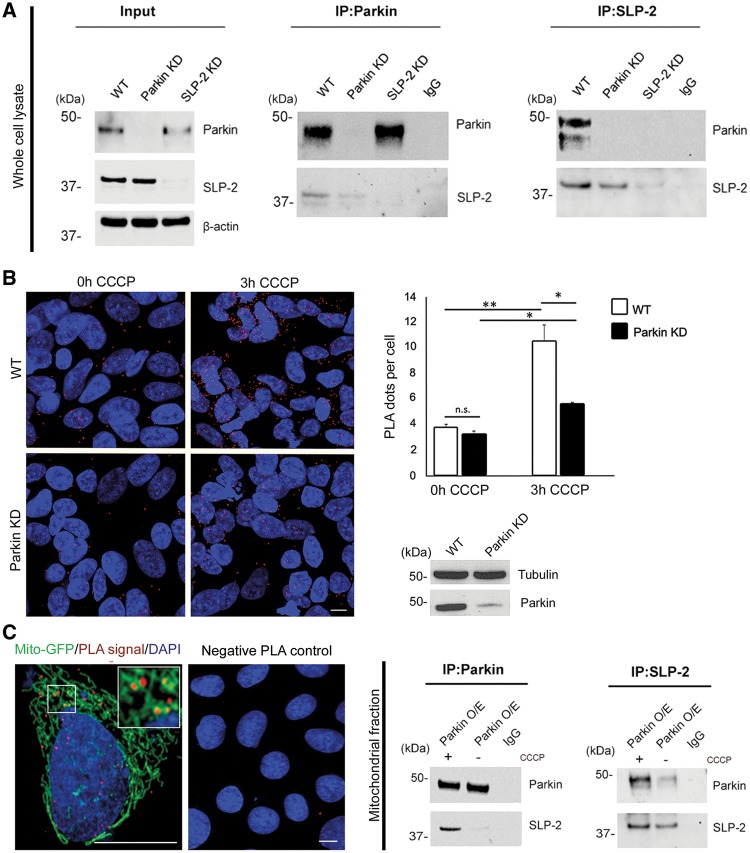
Parkin interacts with mitochondrial SLP-2. (A) Whole cell lysates of untransfected SH-SY5Y cells were subjected to co-immunoprecipitation (IP) with antibodies against Parkin (left panel) and SLP-2 (right panel), followed by Western blotting (WB) of input and IP fractions with the indicated antibodies (the blots were probed consecutively with the antibodies). Cells with knockdown (KD) constructs against Parkin and SLP-2 validate the sensitivity and specificity of the anti-Parkin and anti-SLP-2 antibodies, respectively. IgG was used as negative control for the IPs. Molecular mass markers are in kilodaltons (kDa). (B) SH-SY5Y cells, wild type (WT) and with stable Parkin KD, were processed using the PLA to quantitatively assess the Parkin-SLP-2 interaction under normal culture conditions and after CCCP treatment (3h, 10 µM). The PLA signal is visualized in red, while DAPI-stained nuclei are shown in blue. Exposure to CCCP increased the PLA signal indicating an augmented interaction between the two proteins. The amount of the increase was higher in WT cells (2.8x) compared to Parkin KD cells (1.6x). Two-tailed Students t-test *P < 0.05; **P < 0.01. n.s. = not significant. Scale bar = 7.5 μm. (C) A PLA experiment for Parkin and SLP-2 was co-stained with a mitochondrial marker (green fluorescent protein attached to a mitochondrial leading sequence, mito-GFP) showing a co-localization of the PLA signal with the mitochondria. The specificity of the PLA interaction results was confirmed by performing the experiments with only one of the two primary antibodies. Mitochondrial fractions of SH-SY5Y cells stably overexpressing Parkin, untreated and treated with CCCP, were subjected to IP with anti-Parkin, anti-SLP-2, and anti-IgG antibodies, respectively, followed by WB with the indicated antibodies.