Abstract
People with schizophrenia demonstrate impairments in selective attention, working memory, and executive function. Given the overlap in these constructs, it is unclear if these represent distinct impairments or different manifestations of one higher-order impairment. To examine this question, we administered tasks from the basic cognitive neuroscience literature to measure visual selective attention, working memory capacity, and executive function in 126 people with schizophrenia and 122 healthy volunteers. Patients demonstrated deficits on all tasks with the exception of selective attention guided by strong bottom-up inputs. Although the measures of top-down control of selective attention, working memory, and executive function were all intercorrelated, several sources of evidence indicate that working memory and executive function are separate sources of variance. Specifically, both working memory and executive function independently contributed to the discrimination of group status and independently accounted for variance in overall general cognitive ability as assessed by the MATRICS battery. These two cognitive functions appear to be separable features of the cognitive impairments observed in schizophrenia.
Keywords: attention, cognitive control, working memory
Introduction
Impairments of attention, working memory, and executive control are central features of the cognitive deficits observed in people with schizophrenia (PSZ) and all are plausibly implicated in the broad neuropsychological impairment observed in schizophrenia.1 However, there is substantial overlap in how these terms are used in the literature, and it remains unclear if these represent distinct deficits or differing manifestations of a single underlying deficit in the ability to use internal representations to guide behavior. Although abstract, this question has practical implications for targeting interventions designed to improve cognition.
Some initial distinctions can be established. Working memory (WM) involves the brief maintenance of information in the service of ongoing behavior.2 Two aspects of WM can be separated: (1) the capacity of WM, how much information can be stored, and (2) the ability to manipulate the stored information. In this article, we focus on WM capacity, not manipulation. Attention—specifically selective attention—involves operations that prioritize the processing of a subset of available inputs while suppressing the processing of other inputs.3 Such priority may arise because some stimuli are task-relevant (often called top-down control), or because some stimuli are so salient that they attract attention relatively automatically (often called bottom-up control,4). For this study, we are focusing on only one of the many components of executive function, the ability to maintain and flexibly update the “rules” that apply to the current sets of stimuli and response alternatives.3,5,6 Successful updating necessarily involves inhibition of prior rules so that behavior is guided by the current rule.
While these definitions imply separable systems, the operation of these processes is interactive. For example, the efficient use of WM requires the use of selective attention so that storage capacity is devoted to task-relevant information.7 Similarly, the operation of selective attention requires the maintenance of a search template/rule that biases perceptual systems so that task-relevant representations gain a competitive advantage over irrelevant representations for further processing.8 The case becomes more complicated with executive control. Executive control requires active maintenance of rules to bias perceptual processing/response selection and the ability to update rules in the face of changing circumstances. Executive control is realized through an integration of selective attention and WM in the service of behavioral goals. Thus, these processes are interactive, and impairments in one will impact the other. However, this does not mean that there are no practical distinctions among attention, WM, and executive control. By analogy, the heart, lungs, and vasculature are densely interactive and yet are clearly separable.
The goal of this article is to examine the relationship between these constructs and how they relate to diagnostic status and general cognitive ability as assessed by the MATRICS battery which is widely used to characterize cognitive function in PSZ.9 To assess selective attention, we used a visual search paradigm where PSZ have difficulty using top-down control to guide search, whereas bottom-up control of search is intact.10 Importantly, the search task imposed minimal WM or executive control demands because as the search target remained constant across trials. To assess WM, we used a variant of visual change detection (called change localization), where PSZ show marked capacity deficits that are highly correlated with broad cognitive performance.11,12 The WM displays did not include any distractors, minimizing the need for selective attention, and the task rules remained constant over trials, minimizing demands on executive control.
To measure executive function, we used a variant of the AX CPT called the 1–2 AX CPT.13 In this task, participants see a context cue (either a 1 or a 2) followed by a series of letters. The context cue “1” indicates that an X following, an A is the target whereas a context cue of “2” indicates that a Y following a B is the target for that series. This task puts a premium on the ability to maintain and update rules: the same letter sequence is a target following one context cue and a distractor following the alternative context cue, and this sequence must be maintained to guide a series of target vs nontarget decisions. How are the WM demands different in change localization vs the 1–2 AX CPT? Change localization provides an estimate of how much information a person is capable of storing in WM. In the 1–2 AX CPT, the demand is to dynamically update and maintain rules to guide the use of WM itself: the context cue must be maintained and applied to additional items (ie, the A cue), which must then be maintained in order to respond to the letter (an X or a Y). Thus, although 1–2 AX CPT requires WM, it stresses updating processes, whereas change localization stresses storage capacity.
Even using these refined measures, we expected all three tasks to be modestly intercorrelated in both groups, because it is virtually impossible to design tasks that are fully process-pure and the three cognitive functions studied here are likely involved in most, if not all, intentional behavior. However, we expected that some measures would cluster together more strongly and that some tasks would be more sensitive to diagnostic group. First, because the visual search task was designed to be independent of updating and WM storage, we expected that visual search performance would be weakly related to change localization and the 1–2 AX CPT. Second, because the 1–2 AX CPT task does require WM storage, we expected that performance of this task would be modestly correlated with change localization. However, because the 1–2 AX CPT involves additional updating operations, we expected that these two tasks would exhibit independence in discriminating diagnosis. We did not expect visual search performance to contribute to group discrimination as we previously observed larger between group effects for WM capacity than for top-down visual search slope.9,10
Method
Participants
Over all, 126 people who met Diagnostic and Statistical Manual of Mental Disorders (4th ed.,14) criteria for schizophrenia (n = 105) or schizoaffective disorder (n = 21) were recruited from the outpatient research program of the Maryland Psychiatric Research Center and other local clinics. Diagnosis was established using a best estimate approach combining information from medical records and the results of a Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) Diagnosis.15 The PSZ were clinically stable, receiving the same medication, at the same dose for at least 4 weeks prior to study participation (see table S1 in Supplementary material for details).
Over all, 122 healthy comparison (HC) participants were recruited from the Baltimore area via random digit dialing and internet advertisements and were screened using the Structured Clinical Interview for DSM-IV Axis I and the Structured Clinical Interview for DSM-IV Axis II.15,16 The HCs were free from a current or past history of major psychiatric illness and denied a family history of psychotic disorders in first-degree relatives.
All participants provided written informed consent for a protocol approved by the University of Maryland, Baltimore IRB.
Demographic features are shown in table 1. PSZ completed fewer years of education and scored significantly lower on the Wechsler Test of Adult Reading (WTAR17), the Wide Range Achievement Test Reading subtest (WRAT-418), Wechsler Abbreviated Scale of Intelligence (WASI,19), and the MATRICS Consensus Cognitive Battery composite score (MCCB9).
Table 1.
Participant Characteristics
| Characteristic | SZ | HC | Group Comparisons |
|---|---|---|---|
| N | 126 | 122 | |
| Age | 42.62 (10.86) | 41.40 (11.46) | t = −0.86, P = .39 |
| Education years completed | 12.63 (2.25) | 14.87 (2.07) | t = 8.08, P < .01 |
| Maternal education | 13.17 (2.92) | 13.74 (2.39) | t = 1.63, P = .10 |
| Paternal education | 13.74 (3.67) | 13.54 (3.05) | t = −0.45, P = .65 |
| Gender (% male) | 66 | 60 | χ2 = 2.76, P = .25 |
| Race (% Caucasian) | 59 | 57 | χ2 = 0.00, P = .95 |
| Cognitive performance | |||
| WASI | 95.98 (14.21) | 115.19 (11.20) | t = 11.49, P < .001 |
| WRAT | 94.99 (13.17) | 106.70 (13.52) | t = 6.71, P < .001 |
| WTAR | 97.93 (15.41) | 109.72 (12.58) | t = 6.43, P < .001 |
| MCCB | 29.73 (13.33) | 52.18 (10.72) | t = 14.46, P < .001 |
Note: WASI, Wechsler Abbreviated Scale of Intelligence; WRAT, Wide Range Achievement Test Reading subtest; WTAR, Wechsler Test of Adult Reading; MCCB, MATRICS Consensus Cognitive Battery composite score.
Visual Search Stimuli and Procedure
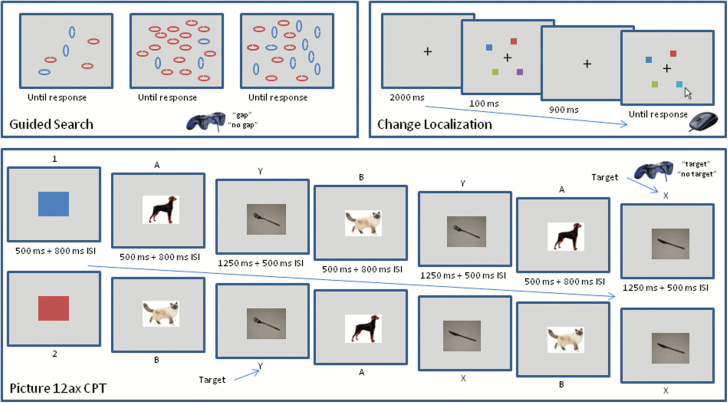
Participants searched for an oval target that was defined by a conjunction of color (red or blue) and orientation (horizontal and vertical) and made a decision about whether the oval had a gap present or not (see figure 1, top left). Every display contained a target that required a gap/no-gap response as well as 5, 11, or 17 distractors. Each distractor shared either the same color or the same orientation as the target, while the other dimension differed from the target. Participants were instructed to restrict their search to items that shared the to-be-attended color. There were a total of 120 trials, 60 Attend-3, and 60 Attend-half trials that were randomly intermixed. On the Attend-3 trials, three of the stimuli (including the target) shared the attended color, regardless of the total number of items in the display. At larger set sizes, the three attended-feature items popped out from the display in a bottom-up manner, reducing the need for top-down control. In the Attend-half trials, half of the items contained the attended color and half contained the other color. In this condition, the attended and unattended items are equally salient, which emphasizes the need for top-down control of attention. Search efficiency was quantified as the slope of the function relating reaction time to set size, which assesses the amount of time needed to process each additional item in the search array. See Supplementary material for additional details.
Fig. 1.
Task illustrations. Top left: Visual Search Displays. In all three panels, the target is a blue vertical oval with a gap on the right or left. When shown with three red distracters (left panel) the Attend-Half and Attend-3 displays fully overlap. When the three blue ovals are the only blue items (Attend-3, middle panel), they attract attention automatically, whereas in the Attend-half (right panel), search requires top-down control. Top right: time-line of the change localization task (not drawn to scale). On every trial the color of one of the items is different on the test array than it was in the sample array. Bottom: examples of the 1–2 AX CPT trial structure, including both alphanumeric and picture stimuli for illustration. Participants only saw the picture stimuli. The two target sequences are 1-AX and 2-BY.
WM Change Localization Stimuli and Task
Visual WM capacity was assessed with a 60-trial change localization task. As illustrated in figure 1, top right, participants were presented with a sample array of four colored squares for 100 ms. After a 900-ms delay during which only the fixation cross was shown, participants were presented with a test array that was identical to the sample array except that one color had changed to a value that had not been present in the sample array. The task was to indicate which item had changed color. WM capacity (termed K) was quantified by multiplying the percentage of correct responses by the array size of 4. See Supplementary material for additional details.
1–2 AX CPT Stimuli and Procedure
This task is designed to challenge executive control by presenting sequences of trials in which the same stimulus pairs could be targets or distractors depending on a context cue that was presented at the beginning of the trial. We implemented the 1–2 AX CPT using color patches and photographs of dogs, cats, knives, and forks (see figure 1, bottom). Red and blue patches were used in place of 1 and 2; dog and cat were used in place of A and B; and knife and fork were used in place of X and Y. Thus, a trial would consist of a seven-item sequence like red-dog-knife-cat-knife-cat-fork. The presentation of these seven items in sequence constitutes a single “trial”. The same dog, cat, knife, and fork photos were used on every trial. See Supplementary material for additional details.
Participants were asked to make a target/nontarget choice for each stimulus on a trial (including the context cues). Across trials, there were two potential targets: the red square context cue indicated that the cat-fork sequence was the target, and the blue square context cue indicated that the dog-knife sequence was the target. The main task consisted of 66 trials, with a trial defined as a color patch cue followed by 3 animal-utensil pairs, for a total of 462 stimulus presentations that required a target/nontarget response.
Participants received extensive practice to ensure task comprehension. Performance was quantified using the signal detection measure d’, which jointly considers the rate of correct target detections as well as false alarms, and was calculated averaging over trial types (see Supplementary material for justification).
Results
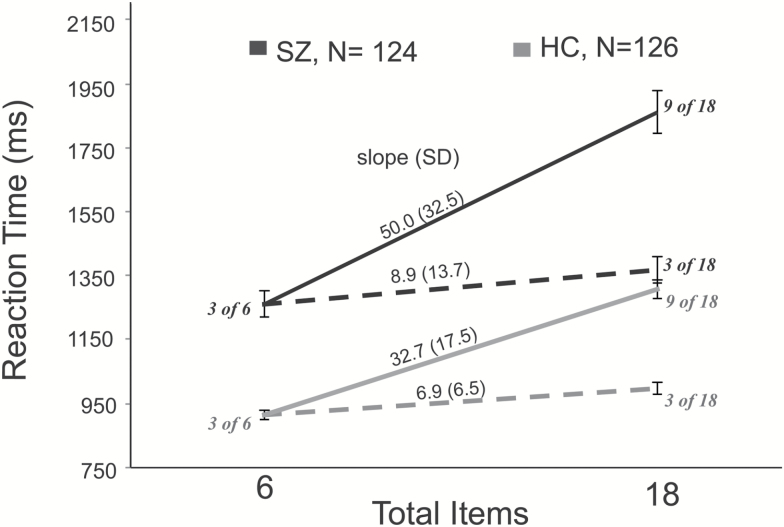
Table 2 summarizes the key variables from all three tasks. Figure 2 shows the data from the visual search tasks broken down into the individual cells of the design. PSZ performed significantly more poorly than HCs on the Attend-half slope measure from visual search, the K measure from change localization, and the overall d’ score from the 1–2 AX CPT task. The groups did not differ on the Attend-3 slope (d = 0.19), replicating our prior finding, and providing evidence that strong bottom-up inputs guide attention normally in PSZ.10
Table 2.
Experimental Task Data for Patients and Healthy Controls
| Characteristic | SZ (N = 126) | HC (N = 122) | Group Comparisons | Effect Sizes |
|---|---|---|---|---|
| Experimental tasks | ||||
| Slope Attend 3a | 8.77 (13.46) | 6.85 (6.55) | t = −1.39, P = .165 | d = 0.19 |
| Slope Attend-halfa | 51.46 (32.79) | 33.42 (17.64) | t = −5.23, P < .001 | d = 0.72 |
| kb | 2.32 (0.56) | 2.88 (0.44)b | t = 8.59, P < .001 | d = 1.12 |
| d’overallc | 2.35 (0.79) | 3.20 (0.49) | t =−9.87, P < .001 | d = 1.33 |
| Target hit rate | 0.85 (0.12) | 0.95 (0.05) | t = 7.50, P < .001 | d = 1.18 |
| 1-BY error rate | 0.15 (0.20) | 0.05 (0.07) | t = −5.07, P < .001 | d = 0.74 |
| 1-AY error rate | 0.14 (0.11) | 0.05 (0.06) | t = −7.72, P < .001 | d = 1.06 |
| 1-BX error rate | 0.08 (0.10) | 0.02 (0.04) | t = −5.45, P < .001 | d = 0.86 |
aData missing for five HC and eight SZ participants.
bData missing for six HC and eight SZ participants.
cData missing for 5 HC and 12 SZ participants.
Fig. 2.
Manual reaction times for HC (in gray) and SZ (in black) from the visual search paradigm. Search slopes are much flatter and did not differ between groups when searching for a target among 3 popout items in an array of 6 or 18 items (dotted lines). Slopes are steeper and the groups differed when searching for a target among 9 of 18 items (solid lines).
We next provide three converging approaches to assess the relationships among these measures. First, we looked at simple pairwise correlations. Next, we used binary stepwise logistic regressions to see how each variable contributed to discriminating between PSZ and HCs. Finally, we used partial correlations to see how the variables that emerged as diagnostic group predictors were predictive of general cognitive ability. To represent general cognitive ability, we did a principal component analysis of the MCCB subtests and used the first principal component (FPC) for all correlational and regression analyses.
Simple Correlations
Table 3 shows the Pearson cross-task correlations. In both groups, the Attend-3 slope showed very modest correlations with K, d’, and the MCCB FPC. It did, however, correlate with the Attend-half slope, likely reflecting shared task variance (because they are extracted from different trials of the same task). The low correlations between Attend-3 slope and the other tasks suggests that the processes related to bottom-up attentional guidance are largely independent of higher-order WM and executive control functions.
Table 3.
Bivariate Correlations Between Each Experimental Measure and the MCCB, Separately for PSZ and HCs
| Att3 Att Half |
Att3 K |
Att3 d’ |
Att3 MCCB |
Att Half K |
Att Half d’ |
Att Half MCCB |
K d’ |
K MCCB |
d’ MCCB |
|
|---|---|---|---|---|---|---|---|---|---|---|
| HC | .45** | −.21* | −.11 | −.11 | −.41** | −.23* | −.43** | .23* | .52** | .44** |
| PSZ | .23* | .04 | .01 | .03 | −.23* | −.41** | −.35** | .53** | .55** | .56** |
Note: PSZ, People with Schizophrenia; HC, healthy control; MCCB, MATRICS Consensus Cognitive Battery composite score; the MCCB score used in the correlation is the first principal component.
* P < .05.
** P < .01.
Significant within-group correlations were found for all combinations of Attend-half slope, d’, K, and MCCB FPC in both groups. In essence, the tasks appear to split into a “bottom-up” group (Attend-3) and a “top- down” group (K and d’), with Attend-half involving both bottom-up and top-down processes. The only correlation that significantly differed in magnitude between PSZ and HC was the correlation of K and d’, where the relationship was stronger in PSZ (z = −2.63, P < .01). This may be the result of PSZ having a wider distribution of scores than do HC, enhancing the ability to detect the relationship. Alternatively, WM capacity and executive function may be more tightly linked in PSZ as a function of illness pathophysiology.
Discrimination Between Groups
We next addressed how the tasks contributed to discriminating the groups using binary logistic regression in the total sample, using backward elimination (given missing data, the N for these analyses included 114 HC and 110 PSZ). From the final model, which only retained the K and d’ scores, the logistic prediction score was calculated for each individual, and the distribution of these scores in PSZ and HC was compared to calculate the area under the receiver operating characteristic (ROC) curve, a measure of how well a diagnostic test separates HC and PSZ; an ROC area of 0.5 represents chance prediction, and one of 1.0 represents perfect prediction. The overall model was significant (χ2 = 96.4, DF = 2, P <. 001), with ROC curve area = 0.85. Looking at predictions by K and d’ alone, the odds of being a PSZ rather than a HC increase 3.5 times for each one unit decrease in K, eg, K = 4 vs K = 3 (95% CI for OR: 1.69, 7.31; ROC curve area = 0.77); for each one unit decrease in d’, the odds of being a PSZ increase 6.1 times (95% CI for OR: 3.14, 11.71; ROC curve area = 0.83). Note, however, that K and d’ are on different scales, so it is not meaningful to directly compare these odds for the two measures.
To determine the robustness of this result, we ran the analysis 10 times, each time removing different, random sets of 10 PSZ and 10 HCs. The classification accuracies of these 10 analyses ranged from 76.9–80.4%, and both K and d’ were significant predictors of group in all 10 regressions. Thus, the result is highly stable and unlikely to be explained by outliers.
Neither slope measure entered any of the models, suggesting that the impairment observed in the Attend-half condition is not predictive of group membership once WM and executive control have been taken into account. To evaluate this directly, we conducted another logistic regression where the Attend-half slope was forced to enter at the first step. We found that Attend-half slope served to correctly classify 61.2% of the participants (P < .001), but both K and d’ remained significant independent predictors when entered at the second step (both P < .001), increasing classification success to 78.6%, evidence that both measures contribute independently of each other and of the variance shared with selective attention.
To examine the contribution of premorbid ability, we first entered the WTAR score in the logistic model and found that only K and d’ remained significant predictors of group (P = .007 and <.001, respectively). To examine the contribution of current cognitive ability, we entered the MCCB FPC at the first step and found that d’, but not K, remained a significant predictor of group ( P = .03). In the final model which only retained MCCB FPC and d’, the odds ratio of MCCB FPC was 1.07 (95% CI: 1.05, 1.09), the odds ratio of d’ was 2.54 (95% CI: 1.24, 5.50), and the area under the ROC curve was 91.45%. These results indicate that K and d’ are significant predictors when premorbid ability is controlled, and d’ remains significant even after current cognitive ability is taken into account. Given that K is presumably an important contributor to cognitive ability, it is not surprising that K was not a significant predictor above and beyond general cognitive ability.
Partial Correlations
Given that K and d’ emerged as significant predictors of group, we next explored whether these two measures independently accounted for variance in MCCB performance. To examine this issue, we performed partial correlations where we removed the influence of each from the other. There were significant partial correlations (P < .001, see table 4) for both d’ and K with the MCCB FPC score in both groups. Thus, there is clear and consistent evidence that both K and d’ independently predict general cognitive ability and diagnostic status. In other words, K and d’ reflect independent sources of variance rather than a single underlying variable.
Table 4.
Partial Correlation Results in Healthy Controls (Top) and People with Schizophrenia (Bottom)
| Covariate | Predictor Variable | MCCB FPC |
|---|---|---|
| d’ | K | 0.48** |
| K | d’ | 0.40** |
| Att_Half | d’ 0.39** | |
| Att_Half | K | 0.43** |
| K | Att_Half | −0.30* |
| d’ | Att_Half | −0.37** |
| d’ | K | 0.38** |
| K | d’ | 0.38** |
| Att_Half | d’ 0.48** | |
| Att_Half | K | 0.53** |
| K | Att_Half | −0.30* |
| d’ | Att_Half | −0.17 |
*P < 0.01.
**P < 0.001.
As seen in table 4, the same relationship with general ability appears to be the case for the Attend-half slope measure, which was not an independent predictor of group. That is, Attend-half slope correlates with the MCCB FPC even after covarying d’ and K in controls, and when covarying K, but not d’, in PSZ.
Discussion
These results offer new insight about the relationships among selective attention, WM capacity, and executive control deficits in PSZ. First, the lack of a deficit in the Attend-3 condition is evidence that PSZ are able to implement selective attention when guided by strong bottom-up salience signals, confirming our previous findings.10 This result is evidence that the cognitive mechanisms that are involved in boosting the processing of relevant items and suppressing the processing of distracters are intact in PSZ, particularly when the relevant items have a bottom-up competitive advantage. This converges with other evidence that PSZ can focus attention onto relevant sources of information and filter distractors under conditions that do not stress top-down control mechanisms.20–22
In contrast, PSZ performed significantly worse than HCs on the Attend-half, K, and d’ measures. These measures were intercorrelated and all correlated with the MCCB. However, when considering the between-group analyses, it becomes evident that these measures reflect different aspects of cognitive impairment in schizophrenia. The Attend-half measure did not enter any of the logistic models, and when Attend-half was forced to enter the model first, K and d’ remained significant predictors of group. It is possible that the patient deficit in the Attend-half measure may be a consequence of deficits in WM and executive control processes, so the Attend-half measure did not contribute any unique variance to predict group membership.
The relationship between WM and executive control is complex. These measures were robustly correlated in PSZ and at lower (but significant) levels in controls. It is easy to imagine how capacity limitations could undermine control: when capacity is too limited, it will be difficult to maintain the rules that are needed to guide behavior. More PSZ than HC likely have capacity below the level needed to mediate executive control, perhaps explaining the higher correlation in PSZ than in HCs. However, if this was the sole source of executive impairment in PSZ, K should have been the only variable to emerge from the logistic models and the partial correlations. Instead, K and d’ independently predicted both group membership and neuropsychological performance. Thus, capacity reduction does not fully account for executive control deficits in PSZ. Similarly, one could imagine that control impairments would limit the ability to selectively store and use information in WM, but the fact that the K and d’ measures showed independence indicates that reduced storage capacity is not solely a consequence of impaired executive control. This conclusion converges with previous research showing that many aspects of executive control over WM encoding are intact in PSZ.23–25
These results have implications for assessment and cognitive remediation. Most simply, WM, and executive control are separable processes and separable deficits in PSZ, a conclusion that likely generalizes to other patient populations. Both should be evaluated in a comprehensive cognitive assessment, and both should be considered treatment targets.
These results are relevant to the debate about whether the cognitive impairment in PSZ is best understood as reflecting the impact of specific deficits or whether it is generalized,26–29 perhaps arising in part from lapses in task engagement that would have a deleterious impact on nearly every task. Our finding of deficits across the Attend-half, K, and d’ measures, their intercorrelations, and their correlations with the MCCB, appear to be consistent with the general deficit view. However, the logistic regression and partial correlation results provide strong evidence for partially separable WM and executive deficits. Note, our findings that d’ remains a predictor of group after controlling for the MCCB FPC differs from a recent article from the BSNIP consortium using different general cognition and executive function measures30 and additional evidence is needed to determine how reliably measures of executive control are related to diagnosis when the contribution of general cognitive ability is controlled. The present results suggest that experimental tasks designed to maximally isolate specific cognitive abilities can define separable aspects of cognitive dysfunction in PSZ.
Our conclusions need to be considered in light of two key limitations. First, it is possible that the poor discriminability of the visual search measures reflects their measurement properties rather than the nature of selective attention, per se. Second, we utilized one measure per construct to minimize assessment time, which makes it difficult to separate method variance error from variance associated with the latent variable of interest. Thus, the use of multiple measures per construct would provide additional precision. That said, the current results suggest that WM change localization and the 1–2 AX CPT may be valuable tools to use in further investigations of the structure of cognitive impairment in PSZ.
Supplementary Material
Supplementary material is available at Schizophrenia Bulletin online.
Funding
This work was supported by NIMH (R01 MH065034).
Acknowledgments
The authors appreciate the efforts of Jackie Kiwanuka, Sharon August, Leeka Hubzin, Samual Kaiser, and Alex Harvey. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Supplementary Material
References
- 1. Lesh TA, Niendam TA, Minzenberg MJ, Carter CS. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology. 2011;36:316–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baddeley A D. Working Memory. Oxford, UK: Clarendon; 1986. [Google Scholar]
- 3. Luck SJ, Gold JM. The construct of attention in schizophrenia. Biol Psychiatry. 2008;64:34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. [DOI] [PubMed] [Google Scholar]
- 5. Meyer DE, Kieras DE. A computational theory of executive cognitive processes and multiple-task performance: part 1. Basic mechanisms. Psychol Rev. 1997;104:3–65. [DOI] [PubMed] [Google Scholar]
- 6. Kerns JG, Nuechterlein KH, Braver TS, Barch DM. Executive functioning component mechanisms and schizophrenia. Biol Psychiatry. 2008;64:26–33. [DOI] [PubMed] [Google Scholar]
- 7. Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438:500–503. [DOI] [PubMed] [Google Scholar]
- 8. Carlisle NB, Arita JT, Pardo D, Woodman GF. Attentional templates in visual working memory. J Neurosci. 2011;31:9315–9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nuechterlein KH, Green MF.. MATRICS Consensus Cognitive Battery Manual. Los Angeles, CA: MATRICS Assessment, Inc; 2006. [Google Scholar]
- 10. Gold JM, Fuller RL, Robinson BM, Braun EL, Luck SJ. Impaired top-down control of visual search in schizophrenia. Schizophr Res. 2007;94:148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnson MJ, McMahon RP, Robinson BM et al. . The relationship between working memory capacity and broad measures of cognitive ability in healthy young adults and people with schizophrenia. Neuropscyhology. 2013;27:220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leonard CJ, Kaiser ST, Robinson BM et al. . Toward the neural mechanisms of reduced working memory capacity in schizophrenia. Cereb Cortex. 2013;23:1582–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frank MJ, Loughry B, O’Reilly RC. Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn Affect Behav Neurosci. 2001;1:137–160. [DOI] [PubMed] [Google Scholar]
- 14. American-Psychiatric-Association (ed.). Diagnostic and Statistical Manual of Mental Disorders (4th, Text Revision Ed.). Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 15. First MB, Spitzer RL, Gibbon M, Williams JBW.. Structured Clinical Interview for DSM-IV- Axis I Disorders (SCID-I).Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 16. Pfoh B, Blum N, Zimmerman M.. Structured Interview for DSM-IV Personality (SID-P). Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 17. Wechsler D. Wechsler Test of Adult Reading (WTAR). San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 18. Wilkinson GS, Robertson GJ.. Wide Range Achievement Test 4: Professional Manual.Lutz, FL: Psychological Assessment Resources, Inc; 2006. [Google Scholar]
- 19. Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI). San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 20. Luck SJ, Fuller RL, Braun EL, Robinson B, Summerfelt A, Gold JM. The speed of visual attention in schizophrenia: electrophysiological and behavioral evidence. Schizophr Res. 2006;85:174–195. [DOI] [PubMed] [Google Scholar]
- 21. Elahipanah A, Christensen BK, Reingold EM. Attentional guidance during visual search among patients with schizophrenia. Schizophr Res. 2011;131:224–230. [DOI] [PubMed] [Google Scholar]
- 22. Elshaikh AA, Sponheim SR, Chafee MV, MacDonald AW. Spatial attentional control is not impaired in schizophrenia: dissociating specific deficits from generalized impairments. J Abnorm Psychol. 2015;124:302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gold JM, Fuller RL, Robinson BM, McMahon RP, Braun EL, Luck SJ. Intact attentional control of working memory encoding in schizophrenia. J Abnorm Psychol. 2006;115:658–673. [DOI] [PubMed] [Google Scholar]
- 24. Erickson M, Hahn B, Leonard C, Robinson B, Luck S, Gold J. Enhanced vulnerability to distraction does not account for working memory capacity reduction in people with schizophrenia. Schizophr Res Cogn. 2014;1:149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hahn B, Hollingworth A, Robinson BM et al. . Control of working memory content in schizophrenia. Schizophr Res. 2012;134:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Green MF, Horan WP, Sugar CA. Has the generalized deficit become the generalized criticism?Schizophr Bull. 2013;39:257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gold JM, Dickinson D. “Generalized cognitive deficit” in schizophrenia: overused or underappreciated?Schizophr Bull. 2013;39:263–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dickinson D, Harvey PD. Systemic hypotheses for generalized cognitive deficits in schizophrenia: a new take on an old problem. Schizophr Bull. 2009;35:403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carter CS, Barch DM, Buchanan RW et al. . Identifying cognitive mechanisms targeted for treatment development in schizophrenia: an overview of the first meeting of the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia Initiative. Biol Psychiatry. 2008;64:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reilly JL, Hill SK, Gold JM et al. . Impaired context processing is attributable to global neuropsychological impairment in schizophrenia and psychotic bipolar disorder. Schizophr Bull. 2017;43:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.